Multiplexed CRISPR-Cas9-Based Genome Editing of Rhodosporidium toruloides
- PMID: 30894433
- PMCID: PMC6429044
- DOI: 10.1128/mSphere.00099-19
Multiplexed CRISPR-Cas9-Based Genome Editing of Rhodosporidium toruloides
Abstract
Microbial production of biofuels and bioproducts offers a sustainable and economic alternative to petroleum-based fuels and chemicals. The basidiomycete yeast Rhodosporidium toruloides is a promising platform organism for generating bioproducts due to its ability to consume a broad spectrum of carbon sources (including those derived from lignocellulosic biomass) and to naturally accumulate high levels of lipids and carotenoids, two biosynthetic pathways that can be leveraged to produce a wide range of bioproducts. While R. toruloides has great potential, it has a more limited set of tools for genetic engineering relative to more advanced yeast platform organisms such as Yarrowia lipolytica and Saccharomyces cerevisiae Significant advancements in the past few years have bolstered R. toruloides' engineering capacity. Here we expand this capacity by demonstrating the first use of CRISPR-Cas9-based gene disruption in R. toruloides Transforming a Cas9 expression cassette harboring nourseothricin resistance and selecting transformants on this antibiotic resulted in strains of R. toruloides exhibiting successful targeted disruption of the native URA3 gene. While editing efficiencies were initially low (0.002%), optimization of the cassette increased efficiencies 364-fold (to 0.6%). Applying these optimized design conditions enabled disruption of another native gene involved in carotenoid biosynthesis, CAR2, with much greater success; editing efficiencies of CAR2 deletion reached roughly 50%. Finally, we demonstrated efficient multiplexed genome editing by disrupting both CAR2 and URA3 in a single transformation. Together, our results provide a framework for applying CRISPR-Cas9 to R. toruloides that will facilitate rapid and high-throughput genome engineering in this industrially relevant organism.IMPORTANCE Microbial biofuel and bioproduct platforms provide access to clean and renewable carbon sources that are more sustainable and environmentally friendly than petroleum-based carbon sources. Furthermore, they can serve as useful conduits for the synthesis of advanced molecules that are difficult to produce through strictly chemical means. R. toruloides has emerged as a promising potential host for converting renewable lignocellulosic material into valuable fuels and chemicals. However, engineering efforts to improve the yeast's production capabilities have been impeded by a lack of advanced tools for genome engineering. While this is rapidly changing, one key tool remains unexplored in R. toruloides: CRISPR-Cas9. The results outlined here demonstrate for the first time how effective multiplexed CRISPR-Cas9 gene disruption provides a framework for other researchers to utilize this revolutionary genome-editing tool effectively in R. toruloides.
Keywords: CAR2; CRISPR-Cas9; Rhodosporidium toruloides; URA3; genome engineering; multiplexed; tRNA.
Figures
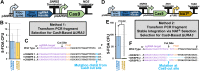
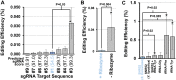
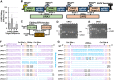
References
-
- Li Y, Zhao ZK, Bai F. 2007. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb Technol 41:312–317. doi: 10.1016/j.enzmictec.2007.02.008. - DOI
-
- Yaegashi J, Kirby J, Ito M, Sun J, Dutta T, Mirsiaghi M, Sundstrom ER, Rodriguez A, Baidoo E, Tanjore D, Pray T, Sale K, Singh S, Keasling JD, Simmons BA, Singer SW, Magnuson JK, Arkin AP, Skerker JM, Gladden JM. 2017. Rhodosporidium toruloides: a new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts. Biotechnol Biofuels 10:241. doi: 10.1186/s13068-017-0927-5. - DOI - PMC - PubMed
-
- Chaturvedi S, Bhattacharya A, Khare SK. 2018. Trends in oil production from oleaginous yeast using biomass: biotechnological potential and constraints. Appl Biochem Microbiol 54:361–369. doi: 10.1134/S000368381804004X. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
