Tomato MYB21 Acts in Ovules to Mediate Jasmonate-Regulated Fertility
- PMID: 30894458
- PMCID: PMC6533027
- DOI: 10.1105/tpc.18.00978
Tomato MYB21 Acts in Ovules to Mediate Jasmonate-Regulated Fertility
Abstract
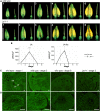
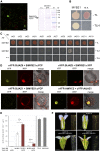
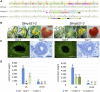
The function of the plant hormone jasmonic acid (JA) in the development of tomato (Solanum lycopersicum) flowers was analyzed with a mutant defective in JA perception (jasmonate-insensitive1-1, jai1-1). In contrast with Arabidopsis (Arabidopsis thaliana) JA-insensitive plants, which are male sterile, the tomato jai1-1 mutant is female sterile, with major defects in female development. To identify putative JA-dependent regulatory components, we performed transcriptomics on ovules from flowers at three developmental stages from wild type and jai1-1 mutants. One of the strongly downregulated genes in jai1-1 encodes the MYB transcription factor SlMYB21. Its Arabidopsis ortholog plays a crucial role in JA-regulated stamen development. SlMYB21 was shown here to exhibit transcription factor activity in yeast, to interact with SlJAZ9 in yeast and in planta, and to complement Arabidopsis myb21-5 To analyze SlMYB21 function, we generated clustered regularly interspaced short palindromic repeats(CRISPR)/CRISPR associated protein 9 (Cas9) mutants and identified a mutant by Targeting Induced Local Lesions in Genomes (TILLING). These mutants showed female sterility, corroborating a function of MYB21 in tomato ovule development. Transcriptomics analysis of wild type, jai1-1, and myb21-2 carpels revealed processes that might be controlled by SlMYB21. The data suggest positive regulation of JA biosynthesis by SlMYB21, but negative regulation of auxin and gibberellins. The results demonstrate that SlMYB21 mediates at least partially the action of JA and might control the flower-to-fruit transition. .
© 2019 American Society of Plant Biologists. All rights reserved.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

