Two Coselected Distal Mutations in HIV-1 Reverse Transcriptase (RT) Alter Susceptibility to Nonnucleoside RT Inhibitors and Nucleoside Analogs
- PMID: 30894467
- PMCID: PMC6532099
- DOI: 10.1128/JVI.00224-19
Two Coselected Distal Mutations in HIV-1 Reverse Transcriptase (RT) Alter Susceptibility to Nonnucleoside RT Inhibitors and Nucleoside Analogs
Abstract
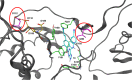
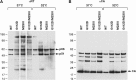
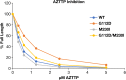
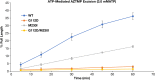
Two mutations, G112D and M230I, were selected in the reverse transcriptase (RT) of human immunodeficiency virus type 1 (HIV-1) by a novel nonnucleoside reverse transcriptase inhibitor (NNRTI). G112D is located near the HIV-1 polymerase active site; M230I is located near the hydrophobic region where NNRTIs bind. Thus, M230I could directly interfere with NNRTI binding but G112D could not. Biochemical and virological assays were performed to analyze the effects of these mutations individually and in combination. M230I alone caused a reduction in susceptibility to NNRTIs, while G112D alone did not. The G112D/M230I double mutant was less susceptible to NNRTIs than was M230I alone. In contrast, both mutations affected the ability of RT to incorporate nucleoside analogs. We suggest that the mutations interact with each other via the bound nucleic acid substrate; the nucleic acid forms part of the polymerase active site, which is near G112D. The positioning of the nucleic acid is influenced by its interactions with the "primer grip" region and could be influenced by the M230I mutation.IMPORTANCE Although antiretroviral therapy (ART) is highly successful, drug-resistant variants can arise that blunt the efficacy of ART. New inhibitors that are broadly effective against known drug-resistant variants are needed, although such compounds might select for novel resistance mutations that affect the sensitivity of the virus to other compounds. Compound 13 selects for resistance mutations that differ from traditional NNRTI resistance mutations. These mutations cause increased sensitivity to NRTIs, such as AZT.
Keywords: drug resistance; nonnucleoside RT inhibitor; nucleic acid repositioning; nucleoside analogs; nucleoside inhibitor.
Copyright © 2019 Boyer et al.
Figures






References
-
- Janssen PA, Lewi PJ, Arnold E, Daeyaert F, de Jonge M, Heeres J, Koymans L, Vinkers M, Guillemont J, Pasquier E, Kukla M, Ludovici D, Andries K, de Bethune MP, Pauwels R, Das K, Clark AD Jr, Frenkel YV, Hughes SH, Medaer B, De Knaep F, Bohets H, De Clerck F, Lampo A, Williams P, Stoffels P. 2005. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2- pyrimidinyl]amino]benzonitrile (R278474, rilpivirine). J Med Chem 48:1901–1909. doi: 10.1021/jm040840e. - DOI - PubMed
-
- Das K, Bauman JD, Clark AD Jr, Frenkel YV, Lewi PJ, Shatkin AJ, Hughes SH, Arnold E. 2008. High-resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: strategic flexibility explains potency against resistance mutations. Proc Natl Acad Sci U S A 105:1466–1471. doi: 10.1073/pnas.0711209105. - DOI - PMC - PubMed
-
- Azijn H, Tirry I, Vingerhoets J, de Bethune MP, Kraus G, Boven K, Jochmans D, Van Craenenbroeck E, Picchio G, Rimsky LT. 2010. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother 54:718–727. doi: 10.1128/AAC.00986-09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

