The N Terminus of Human Cytomegalovirus Glycoprotein O Is Important for Binding to the Cellular Receptor PDGFRα
- PMID: 30894468
- PMCID: PMC6532092
- DOI: 10.1128/JVI.00138-19
The N Terminus of Human Cytomegalovirus Glycoprotein O Is Important for Binding to the Cellular Receptor PDGFRα
Abstract
The human cytomegalovirus (HCMV) glycoprotein complex gH/gL/gO is required for the infection of cells by cell-free virions. It was recently shown that entry into fibroblasts depends on the interaction of gO with the platelet-derived growth factor receptor alpha (PDGFRα). This interaction can be blocked with soluble PDGFRα-Fc, which binds to HCMV virions and inhibits entry. The aim of this study was to identify parts of gO that contribute to PDGFRα binding. In a systematic mutational approach, we targeted potential interaction sites by exchanging conserved clusters of charged amino acids of gO with alanines. To screen for impaired interaction with PDGFRα, virus mutants were tested for sensitivity to inhibition by soluble PDGFRα-Fc. Two mutants with mutations within the N terminus of gO (amino acids 56 to 61 and 117 to 121) were partially resistant to neutralization. To validate whether these mutations impair interaction with PDGFRα-Fc, we compared binding of PDGFRα-Fc to mutant and wild-type virions via quantitative immunofluorescence analysis. PDGFRα-Fc staining intensities were reduced by 30% to 60% with mutant virus particles compared to wild-type particles. In concordance with the reduced binding to the soluble receptor, virus penetration into fibroblasts, which relies on binding to the cellular PDGFRα, was also reduced. In contrast, PDGFRα-independent penetration into endothelial cells was unaltered, demonstrating that the phenotypes of the gO mutant viruses were specific for the interaction with PDGFRα. In conclusion, the mutational screening of gO revealed that the N terminus of gO contributes to efficient spread in fibroblasts by promoting the interaction of virions with its cellular receptor.IMPORTANCE The human cytomegalovirus is a highly prevalent pathogen that can cause severe disease in immunocompromised hosts. Currently used drugs successfully target the viral replication within the host cell, but their use is restricted due to side effects and the development of resistance. An alternative approach is the inhibition of virus entry, for which understanding the details of the initial virus-cell interaction is desirable. As binding of the viral gH/gL/gO complex to the cellular PDGFRα drives infection of fibroblasts, this is a potential target for inhibition of infection. Our mutational mapping approach suggests the N terminus as the receptor binding portion of the protein. The respective mutants were partially resistant to inhibition by PDGFRα-Fc but also attenuated for infection of fibroblasts, indicating that such mutations have little if any benefit for the virus. These findings highlight the potential of targeting the interaction of gH/gL/gO with PDGFRα for therapeutic inhibition of HCMV.
Keywords: cytomegalovirus; glycoproteins; receptors; virus entry.
Copyright © 2019 American Society for Microbiology.
Figures





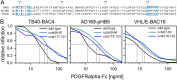
Similar articles
-
Influence of Human Cytomegalovirus Glycoprotein O Polymorphism on the Inhibitory Effect of Soluble Forms of Trimer- and Pentamer-Specific Entry Receptors.J Virol. 2020 Jul 1;94(14):e00107-20. doi: 10.1128/JVI.00107-20. Print 2020 Jul 1. J Virol. 2020. PMID: 32350071 Free PMC article.
-
Human cytomegalovirus TR strain glycoprotein O acts as a chaperone promoting gH/gL incorporation into virions but is not present in virions.J Virol. 2010 Mar;84(5):2597-609. doi: 10.1128/JVI.02256-09. Epub 2009 Dec 23. J Virol. 2010. PMID: 20032193 Free PMC article.
-
Loss of the Human Cytomegalovirus US16 Protein Abrogates Virus Entry into Endothelial and Epithelial Cells by Reducing the Virion Content of the Pentamer.J Virol. 2017 May 12;91(11):e00205-17. doi: 10.1128/JVI.00205-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28331097 Free PMC article.
-
Principles for studying in vivo attenuation of virus mutants: defining the role of the cytomegalovirus gH/gL/gO complex as a paradigm.Med Microbiol Immunol. 2015 Jun;204(3):295-305. doi: 10.1007/s00430-015-0405-2. Epub 2015 Mar 18. Med Microbiol Immunol. 2015. PMID: 25782576 Review.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
Cited by
-
Influence of Human Cytomegalovirus Glycoprotein O Polymorphism on the Inhibitory Effect of Soluble Forms of Trimer- and Pentamer-Specific Entry Receptors.J Virol. 2020 Jul 1;94(14):e00107-20. doi: 10.1128/JVI.00107-20. Print 2020 Jul 1. J Virol. 2020. PMID: 32350071 Free PMC article.
-
Human Cytomegalovirus Modifies Placental Small Extracellular Vesicle Composition to Enhance Infection of Fetal Neural Cells In Vitro.Viruses. 2022 Sep 13;14(9):2030. doi: 10.3390/v14092030. Viruses. 2022. PMID: 36146834 Free PMC article.
-
Polymorphic Forms of Human Cytomegalovirus Glycoprotein O Protect against Neutralization of Fibroblast Entry by Antibodies Targeting Epitopes Defined by Glycoproteins H and L.Viruses. 2022 Jul 9;14(7):1508. doi: 10.3390/v14071508. Viruses. 2022. PMID: 35891489 Free PMC article.
-
Cryo-Electron Microscopy Structure and Interactions of the Human Cytomegalovirus gHgLgO Trimer with Platelet-Derived Growth Factor Receptor Alpha.mBio. 2021 Oct 26;12(5):e0262521. doi: 10.1128/mBio.02625-21. Epub 2021 Oct 26. mBio. 2021. PMID: 34700375 Free PMC article.
-
Human cytomegalovirus gH/gL/gO binding to PDGFRα provides a regulatory signal activating the fusion protein gB that can be blocked by neutralizing antibodies.bioRxiv [Preprint]. 2025 Jan 8:2025.01.08.631902. doi: 10.1101/2025.01.08.631902. bioRxiv. 2025. Update in: J Virol. 2025 May 20;99(5):e0003525. doi: 10.1128/jvi.00035-25. PMID: 39829861 Free PMC article. Updated. Preprint.
References
-
- Adler B, Sinzger C. 2013. Cytomegalovirus interstrain variance in cell type tropism, p 297–321. In Reddehase MM, Lemmermann NAW (ed), Cytomegaloviruses: from molecular pathogenesis to intervention. Caister Academic Press, Norfolk, UK.
-
- Boppana SB, Britt WJ. 2013. Synopsis of clinical aspects of human cytomegalovirus disease, p 1–25. In Reddehase MJ, Lemmermann NAW (ed), Cytomegaloviruses: from molecular pathogenesis to intervention. Caister Academic Press, Norfolk, UK.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

