Foot-and-Mouth Disease Virus Antagonizes NOD2-Mediated Antiviral Effects by Inhibiting NOD2 Protein Expression
- PMID: 30894473
- PMCID: PMC6532107
- DOI: 10.1128/JVI.00124-19
Foot-and-Mouth Disease Virus Antagonizes NOD2-Mediated Antiviral Effects by Inhibiting NOD2 Protein Expression
Abstract
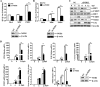
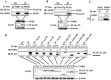
The role of nucleotide-binding oligomerization domain 2 (NOD2) in foot-and-mouth disease virus (FMDV)-infected cells remains unknown. Here, we showed that FMDV infection activated NOD2-mediated beta interferon (IFN-β) and nuclear factor-κB (NF-ĸB) signaling pathways. NOD2 inhibited FMDV replication in the infected cells. FMDV infection triggered NOD2 transcription, while it reduced the abundance of NOD2 protein. Our results revealed that FMDV 2B, 2C, and 3C proteinase (3Cpro) were responsible for the decrease in NOD2 protein levels. 3Cpro is a viral proteinase that can cleave multiple host proteins and limit protein synthesis. Our previous studies determined that FMDV 2B suppressed protein expression of RIG-I and LGP2. Here, we found that 3Cpro and 2B also decreased NOD2 expression. However, this is the first report that 2C induced the reduction of NOD2 protein levels. We determined that both 2B- and 2C-induced decreases in NOD2 were independent of the cleavage of host eukaryotic translation initiation factor 4 gamma (eIF4G), induction of cellular apoptosis, or proteasome, lysosome, and caspase pathways. The interactions between NOD2 and 2B or 2C were observed in the context of viral infection. The carboxyl-terminal amino acids 105 to 114 and 135 to 144 of 2B were essential for the reduction of NOD2, while the residues 105 to 114 were required for the interaction. Amino acids 116 to 260 of the carboxyl terminus of 2C were essential for the interaction, while truncated 2C mutants did not reduce NOD2. These data suggested novel antagonistic mechanisms of FMDV that were mediated by 2B, 2C, and 3Cpro proteins.IMPORTANCE NOD2 was identified as a cytoplasmic viral pattern recognition receptor in 2009. Subsequently, many viruses were reported to activate NOD2-mediated signaling pathways. This study demonstrated that FMDV infection activated NOD2-mediated IFN-β and NF-ĸB signaling pathways. Host cells have developed multiple strategies against viral infection; however, viruses have evolved many strategies to escape host defenses. FMDV has evolved multiple mechanisms to inhibit host type I IFN production. Here, we showed that NOD2 suppressed FMDV replication during viral infection. FMDV 2B, 2C, and 3Cpro decreased NOD2 protein expression by different mechanisms to promote viral replication. This study provided new insight into the immune evasion mechanisms mediated by FMDV and identified 2B, 2C, and 3Cpro as antagonistic factors for FMDV to evade host antiviral responses.
Keywords: 2B; 2C; FMDV; NOD2; antagonistic mechanism.
Copyright © 2019 American Society for Microbiology.
Figures






References
-
- Moffat K, Howell G, Knox C, Belsham GJ, Monaghan P, Ryan MD, Wileman T. 2005. Effects of foot-and-mouth disease virus nonstructural proteins on the structure and function of the early secretory pathway: 2BC but not 3A blocks endoplasmic reticulum-to-Golgi transport. J Virol 79:4382–4395. doi: 10.1128/JVI.79.7.4382-4395.2005. - DOI - PMC - PubMed
-
- Moffat K, Knox C, Howell G, Clark SJ, Yang H, Belsham GJ, Ryan M, Wileman T. 2007. Inhibition of the secretory pathway by foot-and-mouth disease virus 2BC protein is reproduced by coexpression of 2B with 2C, and the site of inhibition is determined by the subcellular location of 2C. J Virol 81:1129–1139. doi: 10.1128/JVI.00393-06. - DOI - PMC - PubMed
-
- Moraes MP, Segundo FD, Dias CC, Pena L, Grubman MJ. 2011. Increased efficacy of an adenovirus-vectored foot-and-mouth disease capsid subunit vaccine expressing nonstructural protein 2B is associated with a specific T cell response. Vaccine 29:9431–9440. doi: 10.1016/j.vaccine.2011.10.037. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

