A Novel Murine Model Expressing a Chimeric mSCARB2/hSCARB2 Receptor Is Highly Susceptible to Oral Infection with Clinical Isolates of Enterovirus 71
- PMID: 30894476
- PMCID: PMC6532076
- DOI: 10.1128/JVI.00183-19
A Novel Murine Model Expressing a Chimeric mSCARB2/hSCARB2 Receptor Is Highly Susceptible to Oral Infection with Clinical Isolates of Enterovirus 71
Abstract
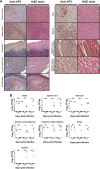
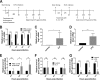
Enterovirus 71 (EV71) infection is generally associated with hand-foot-and-mouth disease (HFMD) and may cause severe neurological disorders and even death. An effective murine oral infection model for studying the pathogenesis of various clinical EV71 isolates is lacking. We developed a transgenic (Tg) mouse that expresses an EV71 receptor, that is, human scavenger receptor class B member 2 (hSCARB2), in a pattern highly similar to that of endogenous murine SCARB2 (mSCARB2) protein. A FLAG-tagged SCARB2 cDNA fragment composed of exons 3 to 12 was inserted into a murine Scarb2 gene-containing bacterial artificial chromosome (BAC) clone, and the resulting transgene was used for establishment of chimeric receptor-expressing Tg mice. Tg mice intragastrically (i.g.) infected with clinical isolates of EV71 showed neurological symptoms, such as ataxia and paralysis, and fatality. There was an age-dependent decrease in susceptibility to viral infection. Pathological characteristics of the infected Tg mice resembled those of encephalomyelitis in human patients. Viral infection was accompanied by microglial activation. Clodronate treatment of the brain slices from Tg mice enhanced viral replication, while lipopolysaccharide treatment significantly inhibited it, suggesting an antiviral role for microglia during EV71 infection. Taken together, this Tg mouse provides a model that closely mimics natural infection for studying EV71 pathogenesis and for evaluating the efficacy of vaccines or other antiviral drugs.IMPORTANCE The availability of a murine model of EV71 infection is beneficial for the understanding of pathogenic mechanisms and the development and assessment of vaccines and antiviral drugs. However, the lack of a murine oral infection model thwarted the study of pathogenesis induced by clinically relevant EV71 strains that are transmitted via the oral-oral or oral-fecal route. Our Tg mice could be intragastrically infected with clinically relevant EV71 strains in an efficient way and developed neurological symptoms and pathological changes strikingly resembling those of human infection. Moreover, these mice showed an age-dependent change in susceptibility that is similar to the human case. This Tg mouse, when combined with the use of other genetically modified mice, potentially contributes to studying the relationship between developmental changes in immunity and susceptibility to virus.
Keywords: SCARB2; enterovirus 71; oral infection.
Copyright © 2019 American Society for Microbiology.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

