Strong triaxial coupling and anomalous Poisson effect in collagen networks
- PMID: 30894480
- PMCID: PMC6452734
- DOI: 10.1073/pnas.1815659116
Strong triaxial coupling and anomalous Poisson effect in collagen networks
Abstract
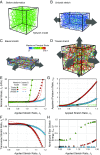
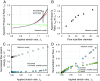
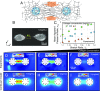
While cells within tissues generate and sense 3D states of strain, the current understanding of the mechanics of fibrous extracellular matrices (ECMs) stems mainly from uniaxial, biaxial, and shear tests. Here, we demonstrate that the multiaxial deformations of fiber networks in 3D cannot be inferred solely based on these tests. The interdependence of the three principal strains gives rise to anomalous ratios of biaxial to uniaxial stiffness between 8 and 9 and apparent Poisson's ratios larger than 1. These observations are explained using a microstructural network model and a coarse-grained constitutive framework that predicts the network Poisson effect and stress-strain responses in uniaxial, biaxial, and triaxial modes of deformation as a function of the microstructural properties of the network, including fiber mechanics and pore size of the network. Using this theoretical approach, we found that accounting for the Poisson effect leads to a 100-fold increase in the perceived elastic stiffness of thin collagen samples in extension tests, reconciling the seemingly disparate measurements of the stiffness of collagen networks using different methods. We applied our framework to study the formation of fiber tracts induced by cellular forces. In vitro experiments with low-density networks showed that the anomalous Poisson effect facilitates higher densification of fibrous tracts, associated with the invasion of cancerous acinar cells. The approach developed here can be used to model the evolving mechanics of ECM during cancer invasion and fibrosis.
Keywords: 3D cell traction force microscopy; fibrous matrices; matrix realignment; tissue swelling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. - PubMed
-
- Das RK, Gocheva V, Hammink R, Zouani OF, Rowan AE. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat Mater. 2016;15:318–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

