The histone demethylase LSD1 promotes renal inflammation by mediating TLR4 signaling in hepatitis B virus-associated glomerulonephritis
- PMID: 30894511
- PMCID: PMC6427019
- DOI: 10.1038/s41419-019-1514-4
The histone demethylase LSD1 promotes renal inflammation by mediating TLR4 signaling in hepatitis B virus-associated glomerulonephritis
Abstract
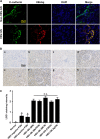
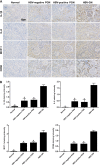
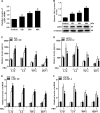
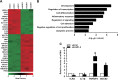
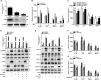
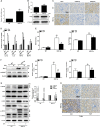
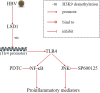
Renal inflammation significantly contributes to the progression of hepatitis B virus (HBV)-associated glomerulonephritis (HBV-GN), but the mechanisms that control its precise regulation remain largely unknown. In this study, we showed that the lysine-specific demethylase 1 (LSD1) was significantly upregulated in renal tissue of HBV-GN patients, and its expression was positively correlated with inflammation. Functionally, LSD1 could promote HBV-induced release of proinflammatory mediators in HK-2 cells, a human renal tubular epithelial (RTE) cell line. Mechanistic investigations suggested that LSD1 directly promoted the transcription of the inflammatory-related gene Tlr4 by eliminating the mono- or di-methylation of H3K9 near its promoter. Knockdown of Lsd1 further inhibited TLR4-NF-κB/JNK signaling cascades, and subsequently decreased HBV-induced production of proinflammatory mediators in HK-2 cells. Co-transfection with Tlr4-expressing plasmids counteracted these effects. Meanwhile, downregulation of abovementioned TLR4-related pathways using small-molecule inhibitors attenuated inflammation. Importantly, LSD1 inhibitor tranylcypromine (TCP) could inhibit TLR4-NF-κB/JNK signaling axis and alleviate renal inflammation in HBV transgenic mice. Taken together, our data identify LSD1 as a novel regulator of renal inflammation and as a potential therapeutic target in HBV-GN.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Lysine-specific demethylase 1 aggravated oxidative stress and ferroptosis induced by renal ischemia and reperfusion injury through activation of TLR4/NOX4 pathway in mice.J Cell Mol Med. 2022 Aug;26(15):4254-4267. doi: 10.1111/jcmm.17444. Epub 2022 Jun 30. J Cell Mol Med. 2022. PMID: 35775122 Free PMC article.
-
The role of the toll-like receptor TLR4 in hepatitis B virus-associated glomerulonephritis.Arch Virol. 2013 Feb;158(2):425-33. doi: 10.1007/s00705-012-1508-3. Epub 2012 Oct 18. Arch Virol. 2013. PMID: 23076739
-
[Toll-like receptor 4 deposition and its significance in hepatitis B virus associated nephropathy].Zhonghua Nei Ke Za Zhi. 2011 Dec;50(12):1008-12. Zhonghua Nei Ke Za Zhi. 2011. PMID: 22333167 Chinese.
-
TCPs: privileged scaffolds for identifying potent LSD1 inhibitors for cancer therapy.Epigenomics. 2016 May;8(5):651-66. doi: 10.2217/epi-2015-0002. Epub 2016 Apr 22. Epigenomics. 2016. PMID: 27102879 Review.
-
Irreversible LSD1 Inhibitors: Application of Tranylcypromine and Its Derivatives in Cancer Treatment.Curr Top Med Chem. 2016;16(19):2179-88. doi: 10.2174/1568026616666160216154042. Curr Top Med Chem. 2016. PMID: 26881714 Review.
Cited by
-
Histone demethylases in the regulation of immunity and inflammation.Cell Death Discov. 2023 Jun 23;9(1):188. doi: 10.1038/s41420-023-01489-9. Cell Death Discov. 2023. PMID: 37353521 Free PMC article. Review.
-
Lysine-specific demethylase 1 deletion reshapes tumour microenvironment to overcome acquired resistance to anti-programmed death 1 therapy in liver cancer.Clin Transl Med. 2025 May;15(5):e70335. doi: 10.1002/ctm2.70335. Clin Transl Med. 2025. PMID: 40356247 Free PMC article.
-
Epigenetic regulation of Toll-like receptors 2 and 4 in kidney disease.J Mol Med (Berl). 2022 Jul;100(7):1017-1026. doi: 10.1007/s00109-022-02218-y. Epub 2022 Jun 15. J Mol Med (Berl). 2022. PMID: 35704060 Review.
-
miR-708-3p targetedly regulates LSD1 to promote osteoblast differentiation of hPDLSCs in periodontitis.Odontology. 2025 Jan;113(1):222-230. doi: 10.1007/s10266-024-00963-9. Epub 2024 Jul 3. Odontology. 2025. PMID: 38961043 Free PMC article.
-
Metabolic Regulation of Hepatitis B Virus Infection in HBV-Transgenic Mice.Metabolites. 2022 Mar 25;12(4):287. doi: 10.3390/metabo12040287. Metabolites. 2022. PMID: 35448475 Free PMC article.
References
-
- Yi Z, Jie YW, Nan Z. The efficacy of anti-viral therapy on hepatitis B virus-associated glomerulonephritis: a systematic review and meta-analysis. Ann. Hepatol. 2011;10:165–173. - PubMed
-
- Takekoshi Y, Tochimaru H, Nagata Y, Itami N. Immunopathogenetic mechanisms of hepatitis B virus-related glomerulopathy. Kidney Int. Suppl. 1991;35:S34–S39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

