Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study
- PMID: 30894515
- PMCID: PMC6426965
- DOI: 10.1038/s41408-019-0196-6
Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study
Abstract
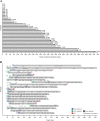
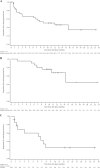
Interim analyses of a phase I study with GSK2857916, an antibody-drug conjugate against B cell maturation antigen, have previously reported a 60% overall response and 7.9 months progression-free survival in relapsed/refractory multiple myeloma (MM). We provide updated safety and efficacy results of the BMA117159 trial following an additional 14 months' follow-up. This open-label, first-in-human, phase I study was conducted at nine centres in the USA, Canada and the UK, and included adults with MM and progressive disease after stem cell transplantation, alkylators, proteasome inhibitors, and immunomodulators. In part 1, the recommended dose of 3.4 mg/kg was identified; in part 2, patients received GSK2857916 3.4 mg/kg once every 3 weeks. Selected part 2 safety/tolerability and efficacy endpoints are reported. Twenty-one (60.0%; 95% confidence interval (CI) 42.1-76.1) of 35 patients achieved partial response or better, including two stringent complete responses and three complete responses. The median progression-free survival was 12 months and median duration of response was 14.3 months. Thrombocytopenia and corneal events were commonly reported; no new safety signals were identified. GSK2857916 was well tolerated and demonstrated a rapid, deep and durable response in heavily pre-treated patients with relapsed/refractory MM, consolidating the interim analyses conclusions that GSK2857916 is a promising treatment for these patients.
Conflict of interest statement
A.D.C. is a consultant for and a member of an advisory board for GlaxoSmithKline and Celgene, is a member of an advisory board for Janssen, Takeda, Oncopeptides, Kite Pharma, Seattle Genetics and Bristol-Myers Squibb, and has received research funding from Bristol-Myers Squibb and Novartis. N.L. has received research funding from GlaxoSmithKline, Takeda, Karyopharm, Sanofi and Amgen and served as consultant for Karyopharm and Amgen. R.P. has received honoraria from Janssen, Takeda, Celgene and Amgen, and travel support to attend meetings from Janssen, Takeda and Celgene. P.G.R. has received research funding from Celgene, Takeda, Oncopeptides and BMS, and is a member of advisory committees for Celgene, Oncopeptides, Janssen and Takeda. S.T. is a consultant for and has received honoraria from Amgen and Celgene, has received honoraria from Takeda and AbbVie, is a consultant for Novartis, and has received research support from Janssen. P.M.V. is a consultant for Amgen, Celgene, Janssen, Bristol-Myers Squibb, Novartis, Takeda, Oncopeptides and Teneo-Bio, and has participated in speakers’ bureaux for Amgen, Celgene and Janssen. I.G., A.H., V.B., J.B.O. and Z.H. are employees of and hold stocks/shares in GlaxoSmithKline. B.R. and E.N.L. declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

