Axial shielding of Pd(II) complexes enables perfect stereoretention in Suzuki-Miyaura cross-coupling of Csp3 boronic acids
- PMID: 30894535
- PMCID: PMC6427018
- DOI: 10.1038/s41467-019-09249-z
Axial shielding of Pd(II) complexes enables perfect stereoretention in Suzuki-Miyaura cross-coupling of Csp3 boronic acids
Abstract
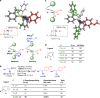
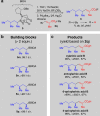
Stereocontrolled Csp3 cross-coupling can fundamentally change the types of chemical structures that can be mined for molecular functions. Although considerable progress in achieving the targeted chemical reactivity has been made, controlling stereochemistry in Csp3 cross-coupling remains challenging. Here we report that ligand-based axial shielding of Pd(II) complexes enables Suzuki-Miyaura cross-coupling of unactivated Csp3 boronic acids with perfect stereoretention. This approach leverages key differences in spatial orientation between competing pathways for stereoretentive and stereoinvertive transmetalation of Csp3 boronic acids to Pd(II). We show that axial shielding enables perfectly stereoretentive cross-coupling with a range of unactivated secondary Csp3 boronic acids, as well as the stereocontrolled synthesis of xylarinic acid B and all of its Csp3 stereoisomers. We expect these ligand design principles will broadly enable the continued search for practical and effective methods for stereospecific Csp3 cross-coupling.
Conflict of interest statement
The University of Illinois has filed patent applications on ligands and methods reported in this manuscript, and some of these have been licensed to REVOLUTION Medicines, a company for which M.D.B. is a founder. All the remaining authors declare no competing interests.
Figures



References
-
- Harris MR, Hanna LE, Greene MA, Moore CE, Jarvo ER. Retention or inversion in stereospecific nickel-catalyzed cross-coupling of benzylic carbamates with arylboronic esters. Control of absolute stereochemistry with an achiral catalyst. J. Am. Chem. Soc. 2013;135:3303–3306. doi: 10.1021/ja311783k. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

