Disturbed flow induces a sustained, stochastic NF-κB activation which may support intracranial aneurysm growth in vivo
- PMID: 30894565
- PMCID: PMC6426999
- DOI: 10.1038/s41598-019-40959-y
Disturbed flow induces a sustained, stochastic NF-κB activation which may support intracranial aneurysm growth in vivo
Abstract
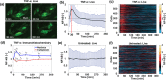
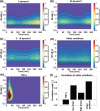
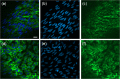
Intracranial aneurysms are associated with disturbed velocity patterns, and chronic inflammation, but the relevance for these findings are currently unknown. Here, we show that (disturbed) shear stress induced by vortices is a sufficient condition to activate the endothelial NF-kB pathway, possibly through a mechanism of mechanosensor de-activation. We provide evidence for this statement through in-vitro live cell imaging of NF-kB in HUVECs exposed to different flow conditions, stochastic modelling of flow induced NF-kB activation and induction of disturbed flow in mouse carotid arteries. Finally, CFD and immunofluorescence on human intracranial aneurysms showed a correlation similar to the mouse vessels, suggesting that disturbed shear stress may lead to sustained NF-kB activation thereby offering an explanation for the close association between disturbed flow and intracranial aneurysms.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

