Moderate Nucleoporin 133 deficiency leads to glomerular damage in zebrafish
- PMID: 30894603
- PMCID: PMC6426968
- DOI: 10.1038/s41598-019-41202-4
Moderate Nucleoporin 133 deficiency leads to glomerular damage in zebrafish
Erratum in
-
Author Correction: Moderate Nucleoporin 133 deficiency leads to glomerular damage in zebrafish.Sci Rep. 2020 Jan 15;10(1):756. doi: 10.1038/s41598-020-57829-7. Sci Rep. 2020. PMID: 31937913 Free PMC article.
-
Publisher Correction: Moderate Nucleoporin 133 deficiency leads to glomerular damage in zebrafish.Sci Rep. 2020 Feb 19;10(1):3326. doi: 10.1038/s41598-020-58959-8. Sci Rep. 2020. PMID: 32075994 Free PMC article.
Abstract
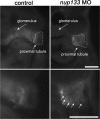
Although structural nuclear pore proteins (nucleoporins) are seemingly required in every cell type to assemble a functional nuclear transport machinery, mutations or deregulation of a subset of them have been associated with specific human hereditary diseases. In particular, previous genetic studies of patients with nephrotic syndrome identified mutations in Nup107 that impaired the expression or the localization of its direct partner at nuclear pores, Nup133. In the present study, we characterized the zebrafish nup133 orthologous gene and its expression pattern during larval development. Using a morpholino-mediated gene knockdown, we show that partial depletion of Nup133 in zebrafish larvae leads to the formation of kidney cysts, a phenotype that can be rescued by co-injection of wild type mRNA. Analysis of different markers for tubular and glomerular development shows that the overall kidney development is not affected by nup133 knockdown. Likewise, no gross defect in nuclear pore complex assembly was observed in these nup133 morphants. On the other hand, nup133 downregulation results in proteinuria and moderate foot process effacement, mimicking some of the abnormalities typically featured by patients with nephrotic syndrome. These data indicate that nup133 is a new gene required for proper glomerular structure and function in zebrafish.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Biallelic Mutations in Nuclear Pore Complex Subunit NUP107 Cause Early-Childhood-Onset Steroid-Resistant Nephrotic Syndrome.Am J Hum Genet. 2015 Oct 1;97(4):555-66. doi: 10.1016/j.ajhg.2015.08.013. Epub 2015 Sep 24. Am J Hum Genet. 2015. PMID: 26411495 Free PMC article.
-
Homozygous splicing mutation in NUP133 causes Galloway-Mowat syndrome.Ann Neurol. 2018 Dec;84(6):814-828. doi: 10.1002/ana.25370. Ann Neurol. 2018. PMID: 30427554
-
Mutations in multiple components of the nuclear pore complex cause nephrotic syndrome.J Clin Invest. 2018 Oct 1;128(10):4313-4328. doi: 10.1172/JCI98688. Epub 2018 Sep 4. J Clin Invest. 2018. PMID: 30179222 Free PMC article.
-
Moonlighting nuclear pore proteins: tissue-specific nucleoporin function in health and disease.Histochem Cell Biol. 2018 Dec;150(6):593-605. doi: 10.1007/s00418-018-1748-8. Epub 2018 Oct 25. Histochem Cell Biol. 2018. PMID: 30361777 Review.
-
Glomerular changes in hereditary single-gene diseases.J Nephrol. 2002 Nov-Dec;15 Suppl 6:S47-56. J Nephrol. 2002. PMID: 12515374 Review.
Cited by
-
A library of sensitive position-specific scoring matrices for high-throughput identification of nuclear pore complex subunits.NAR Genom Bioinform. 2023 Mar 23;5(1):lqad025. doi: 10.1093/nargab/lqad025. eCollection 2023 Mar. NAR Genom Bioinform. 2023. PMID: 36968432 Free PMC article.
-
NUP133 Controls Nuclear Pore Assembly, Transcriptome Composition, and Cytoskeleton Regulation in Podocytes.Cells. 2022 Apr 7;11(8):1259. doi: 10.3390/cells11081259. Cells. 2022. PMID: 35455939 Free PMC article.
-
Nuclear pore complexes in development and tissue homeostasis.Development. 2020 Dec 15;147(23):dev183442. doi: 10.1242/dev.183442. Development. 2020. PMID: 33323374 Free PMC article. Review.
References
-
- Beck M, Hurt E. The nuclear pore complex: understanding its function through structural insight. Nat Rev Mol Cell Biol. 2017;18:73–89. - PubMed
-
- Hezwani M, Fahrenkrog B. The functional versatility of the nuclear pore complex proteins. Semin Cell Dev Biol. 2017;68:2–9. - PubMed
-
- Patrakka J, Tryggvason K. Molecular make-up of the glomerular filtration barrier. Biochem Biophys Res Commun. 2010;396:164–169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

