Long-Lasting Response Changes in Deep Cerebellar Nuclei in vivo Correlate With Low-Frequency Oscillations
- PMID: 30894802
- PMCID: PMC6414422
- DOI: 10.3389/fncel.2019.00084
Long-Lasting Response Changes in Deep Cerebellar Nuclei in vivo Correlate With Low-Frequency Oscillations
Erratum in
-
Corrigendum: Long-Lasting Response Changes in Deep Cerebellar Nuclei in vivo Correlate With Low-Frequency Oscillations.Front Cell Neurosci. 2019 Jul 10;13:313. doi: 10.3389/fncel.2019.00313. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31354433 Free PMC article.
Abstract
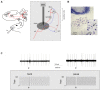
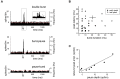
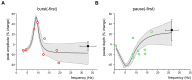
The deep cerebellar nuclei (DCN) have been suggested to play a critical role in sensorimotor learning and some forms of long-term synaptic plasticity observed in vitro have been proposed as a possible substrate. However, till now it was not clear whether and how DCN neuron responses manifest long-lasting changes in vivo. Here, we have characterized DCN unit responses to tactile stimulation of the facial area in anesthetized mice and evaluated the changes induced by theta-sensory stimulation (TSS), a 4 Hz stimulation pattern that is known to induce plasticity in the cerebellar cortex in vivo. DCN units responded to tactile stimulation generating bursts and pauses, which reflected combinations of excitatory inputs most likely relayed by mossy fiber collaterals, inhibitory inputs relayed by Purkinje cells, and intrinsic rebound firing. Interestingly, initial bursts and pauses were often followed by stimulus-induced oscillations in the peri-stimulus time histograms (PSTH). TSS induced long-lasting changes in DCN unit responses. Spike-related potentiation and suppression (SR-P and SR-S), either in units initiating the response with bursts or pauses, were correlated with stimulus-induced oscillations. Fitting with resonant functions suggested the existence of peaks in the theta-band (burst SR-P at 9 Hz, pause SR-S at 5 Hz). Optogenetic stimulation of the cerebellar cortex altered stimulus-induced oscillations suggesting that Purkinje cells play a critical role in the circuits controlling DCN oscillations and plasticity. This observation complements those reported before on the granular and molecular layers supporting the generation of multiple distributed plasticities in the cerebellum following naturally patterned sensory entrainment. The unique dependency of DCN plasticity on circuit oscillations discloses a potential relationship between cerebellar learning and activity patterns generated in the cerebellar network.
Keywords: cerebellum; deep cerebellar nuclei; in vivo electrophysiology; oscillations; plasticity.
Figures




References
-
- Albus J. (1971). The theory of cerebellar function. Math. Biosci. 10 25–61. 10.1016/0025-5564(71)90051-4 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

