Arterial Spin Labeling Reveals Disrupted Brain Networks and Functional Connectivity in Drug-Resistant Temporal Epilepsy
- PMID: 30894811
- PMCID: PMC6414423
- DOI: 10.3389/fninf.2018.00101
Arterial Spin Labeling Reveals Disrupted Brain Networks and Functional Connectivity in Drug-Resistant Temporal Epilepsy
Abstract
Resting-state networks (RSNs) and functional connectivity (FC) have been increasingly exploited for mapping brain activity and identifying abnormalities in pathologies, including epilepsy. The majority of studies currently available are based on blood-oxygenation-level-dependent (BOLD) contrast in combination with either independent component analysis (ICA) or pairwise region of interest (ROI) correlations. Despite its success, this approach has several shortcomings as BOLD is only an indirect and non-quantitative measure of brain activity. Conversely, promising results have recently been achieved by arterial spin labeling (ASL) MRI, primarily developed to quantify brain perfusion. However, the wide application of ASL-based FC has been hampered by its complexity and relatively low robustness to noise, leaving several aspects of this approach still largely unexplored. In this study, we firstly aimed at evaluating the effect of noise reduction on spatio-temporal ASL analyses and quantifying the impact of two ad-hoc processing pipelines (basic and advanced) on connectivity measures. Once the optimal strategy had been defined, we investigated the applicability of ASL for connectivity mapping in patients with drug-resistant temporal epilepsy vs. controls (10 per group), aiming at revealing between-group voxel-wise differences in each RSN and ROI-wise FC changes. We first found ASL was able to identify the main network (DMN) along with all the others generally detected with BOLD but never previously reported from ASL. For all RSNs, ICA-based denoising (advanced pipeline) allowed to increase their similarity with the corresponding BOLD template. ASL-based RSNs were visibly consistent with literature findings; however, group differences could be identified in the structure of some networks. Indeed, statistics revealed areas of significant FC decrease in patients within different RSNs, such as DMN and cerebellum (CER), while significant increases were found in some cases, such as the visual networks. Finally, the ROI-based analyses identified several inter-hemispheric dysfunctional links (controls > patients) mainly between areas belonging to the DMN, right-left thalamus and right-left temporal lobe. Conversely, fewer connections, predominantly intra-hemispheric, showed the opposite pattern (controls < patients). All these elements provide novel insights into the pathological modulations characterizing a "network disease" as epilepsy, shading light on the importance of perfusion-based approaches for identifying the disrupted areas and communications between brain regions.
Keywords: ICA; arterial spin labeling; epilepsy; functional connectivity; perfusion; resting-state.
Figures
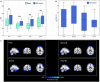




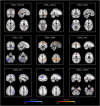

Similar articles
-
Resting state brain function analysis using concurrent BOLD in ASL perfusion fMRI.PLoS One. 2013 Jun 4;8(6):e65884. doi: 10.1371/journal.pone.0065884. Print 2013. PLoS One. 2013. PMID: 23750275 Free PMC article.
-
Functional connectivity in BOLD and CBF data: similarity and reliability of resting brain networks.Neuroimage. 2015 Feb 1;106:111-22. doi: 10.1016/j.neuroimage.2014.11.028. Epub 2014 Nov 21. Neuroimage. 2015. PMID: 25463468 Free PMC article.
-
Noise Reduction in Arterial Spin Labeling Based Functional Connectivity Using Nuisance Variables.Front Neurosci. 2016 Aug 23;10:371. doi: 10.3389/fnins.2016.00371. eCollection 2016. Front Neurosci. 2016. PMID: 27601973 Free PMC article.
-
Characterizing Resting-State Brain Function Using Arterial Spin Labeling.Brain Connect. 2015 Nov;5(9):527-42. doi: 10.1089/brain.2015.0344. Epub 2015 Oct 6. Brain Connect. 2015. PMID: 26106930 Free PMC article. Review.
-
Opposite effects of dopamine and serotonin on resting-state networks: review and implications for psychiatric disorders.Mol Psychiatry. 2020 Jan;25(1):82-93. doi: 10.1038/s41380-019-0406-4. Epub 2019 Apr 5. Mol Psychiatry. 2020. PMID: 30953003 Review.
Cited by
-
Altered cerebral blood flow in patients with unilateral venous pulsatile tinnitus: an arterial spin labeling study.Br J Radiol. 2021 Apr 1;94(1120):20200990. doi: 10.1259/bjr.20200990. Epub 2021 Mar 18. Br J Radiol. 2021. PMID: 33733819 Free PMC article.
-
Disrupted resting-state functional connectivity and network topology in mild traumatic brain injury: an arterial spin labelling study.Brain Commun. 2023 Sep 30;5(5):fcad254. doi: 10.1093/braincomms/fcad254. eCollection 2023. Brain Commun. 2023. PMID: 37829696 Free PMC article.
-
Similar neural pathways link psychological stress and brain-age in health and multiple sclerosis.iScience. 2023 Aug 18;26(9):107679. doi: 10.1016/j.isci.2023.107679. eCollection 2023 Sep 15. iScience. 2023. PMID: 37680475 Free PMC article.
-
Excitation, but not inhibition, of the fastigial nucleus provides powerful control over temporal lobe seizures.J Physiol. 2020 Jan;598(1):171-187. doi: 10.1113/JP278747. Epub 2019 Dec 9. J Physiol. 2020. PMID: 31682010 Free PMC article.
-
Recent Technical Developments in ASL: A Review of the State of the Art.Magn Reson Med. 2022 Nov;88(5):2021-2042. doi: 10.1002/mrm.29381. Epub 2022 Aug 19. Magn Reson Med. 2022. PMID: 35983963 Free PMC article. Review.
References
-
- Alsop D. C., Detre J. A., Golay X., Günther M., Hendriks J., Hernandez-Garcia L., et al. (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Imaging 73 102–116. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

