Osteogenic Differentiation Potential of Human Bone Marrow and Amniotic Fluid-Derived Mesenchymal Stem Cells in Vitro & in Vivo
- PMID: 30894903
- PMCID: PMC6420942
- DOI: 10.3889/oamjms.2019.124
Osteogenic Differentiation Potential of Human Bone Marrow and Amniotic Fluid-Derived Mesenchymal Stem Cells in Vitro & in Vivo
Abstract
Background: Cell therapies offer a promising potential in promoting bone regeneration. Stem cell therapy presents attractive care modality in treating degenerative conditions or tissue injuries. The rationale behind this is both the expansion potential of stem cells into a large cell population size and its differentiation abilities into a wide variety of tissue types, when given the proper stimuli. A progenitor stem cell is a promising source of cell therapy in regenerative medicine and bone tissue engineering.
Aim: This study aimed to compare the osteogenic differentiation and regenerative potentials of human mesenchymal stem cells derived from human bone marrow (hBM-MSCs) or amniotic fluid (hAF-MSCs), both in vitro and in vivo studies.
Subjects and methods: Human MSCs, used in this study, were successfully isolated from two human sources; the bone marrow (BM) and amniotic fluid (AF) collected at the gestational ages of second or third trimesters.
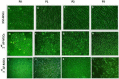
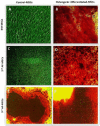
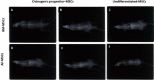
Results: The stem cells derived from amniotic fluid seemed to be the most promising type of progenitor cells for clinical applications. In a pre-clinical experiment, attempting to explore the therapeutic application of MSCs in bone regeneration, Rat lumbar spines defects were surgically created and treated with undifferentiated and osteogenically differentiated MSCs, derived from BM and second trimester AF. Cells were loaded on gel-foam scaffolds, inserted and fixed in the area of the surgical defect. X-Ray radiography follows up, and histopathological analysis was done three-four months post- operation. The transplantation of AF-MSCs or BM-MSCs into induced bony defects showed promising results. The AF-MSCs are offering a better healing effect increasing the likelihood of achieving successful spinal fusion. Some bone changes were observed in rats transplanted with osteoblasts differentiated cells but not in rats transplanted with undifferentiated MSCs. Longer observational periods are required to evaluate a true bone formation. The findings of this study suggested that the different sources; hBM-MSCs or hAF-MSCs exhibited remarkably different signature regarding the cell morphology, proliferation capacity and osteogenic differentiation potential.
Conclusions: AF-MSCs have a better performance in vivo bone healing than that of BM-MSCs. Hence, AF derived MSCs is highly recommended as an alternative source to BM-MSCs in bone regeneration and spine fusion surgeries. Moreover, the usage of gel-foam as a scaffold proved as an efficient cell carrier that showed bio-compatibility with cells, bio-degradability and osteoinductivity in vivo.
Keywords: AF-MSCs; Amniotic fluid; BM-MSCs; Bone marrow; Mesenchymal stem cells; Osteogenic differentiation.
Figures


Similar articles
-
Combination of Human Amniotic Fluid Derived-Mesenchymal Stem Cells and Nano-hydroxyapatite Scaffold Enhances Bone Regeneration.Open Access Maced J Med Sci. 2019 Sep 14;7(17):2739-2750. doi: 10.3889/oamjms.2019.730. eCollection 2019 Sep 15. Open Access Maced J Med Sci. 2019. PMID: 31844430 Free PMC article.
-
Mesenchymal stromal cells from amniotic fluid are less prone to senescence compared to those obtained from bone marrow: An in vitro study.J Cell Physiol. 2018 Nov;233(11):8996-9006. doi: 10.1002/jcp.26845. Epub 2018 Jun 15. J Cell Physiol. 2018. PMID: 29904927
-
Comparison of the neural differentiation potential of human mesenchymal stem cells from amniotic fluid and adult bone marrow.Cell Mol Neurobiol. 2013 May;33(4):465-75. doi: 10.1007/s10571-013-9922-y. Epub 2013 Mar 12. Cell Mol Neurobiol. 2013. PMID: 23478940 Free PMC article.
-
Mesenchymal Stem Cells from Wharton's Jelly and Amniotic Fluid.Best Pract Res Clin Obstet Gynaecol. 2016 Feb;31:30-44. doi: 10.1016/j.bpobgyn.2015.07.006. Epub 2015 Sep 10. Best Pract Res Clin Obstet Gynaecol. 2016. PMID: 26482184 Review.
-
Therapeutic Potential of Amniotic Fluid Derived Mesenchymal Stem Cells Based on their Differentiation Capacity and Immunomodulatory Properties.Curr Stem Cell Res Ther. 2019;14(4):327-336. doi: 10.2174/1574888X14666190222201749. Curr Stem Cell Res Ther. 2019. PMID: 30806325 Review.
Cited by
-
Surface Deformation of Biocompatible Materials: Recent Advances in Biological Applications.Biomimetics (Basel). 2024 Jun 28;9(7):395. doi: 10.3390/biomimetics9070395. Biomimetics (Basel). 2024. PMID: 39056836 Free PMC article. Review.
-
Vancomycin Containing PDLLA and PLGA/β-TCP Inhibit Biofilm Formation but Do Not Stimulate Osteogenic Transformation of Human Mesenchymal Stem Cells.Front Surg. 2022 Jul 1;9:885241. doi: 10.3389/fsurg.2022.885241. eCollection 2022. Front Surg. 2022. PMID: 35846965 Free PMC article.
-
Different phenotypes and chondrogenic responses of human menstrual blood and bone marrow mesenchymal stem cells to activin A and TGF-β3.Stem Cell Res Ther. 2021 Apr 29;12(1):251. doi: 10.1186/s13287-021-02286-w. Stem Cell Res Ther. 2021. PMID: 33926568 Free PMC article.
-
Stem Cells and Acellular Preparations in Bone Regeneration/Fracture Healing: Current Therapies and Future Directions.Cells. 2024 Jun 17;13(12):1045. doi: 10.3390/cells13121045. Cells. 2024. PMID: 38920674 Free PMC article. Review.
-
Regeneration enhanced in critical-sized bone defects using bone-specific extracellular matrix protein.J Biomed Mater Res B Appl Biomater. 2021 Apr;109(4):538-547. doi: 10.1002/jbm.b.34722. Epub 2020 Sep 11. J Biomed Mater Res B Appl Biomater. 2021. PMID: 32915522 Free PMC article.
References
-
- Barry FP, Murphy JM. Mesenchymal stem cells:clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36(4):568–584. https://doi.org/10.1016/j.biocel.2003.11.001 PMid:15010324. - PubMed
-
- Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources:their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792–806. https://doi.org/10.1177/0022034509340867 PMid:19767575 PMCid:PMC2830488. - PMC - PubMed
-
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. https://doi.org/10.1634/stemcells.2005-0342 PMid:16410387. - PubMed
-
- Klingemann H, Matzilevich D, Marchand J. Mesenchymal Stem Cells - Sources and Clinical Applications. Transfus Med Hemother. 2008;35(4):272–277. https://doi.org/10.1159/000142333 PMid:21512642 PMCid:PMC3076359. - PMC - PubMed
-
- Jaquiéry C, Schaeren S, Farhadi J, Mainil-Varlet P, Kunz C, Zeilhofer HF, Heberer M, Martin I. In vitro osteogenic differentiation and in vivo bone-forming capacity of human isogenic jaw periosteal cells and bone marrow stromal cells. Annals of Surgery. 2005;242(6):859–867. https://doi.org/10.1097/01.sla.0000189572.02554.2c PMid:16327496 PMCid:PMC1409890. - PMC - PubMed
LinkOut - more resources
Full Text Sources
