Plasma rich in growth factors stimulates proliferation, migration, and gene expression associated with bone formation in human dental follicle cells
- PMID: 30894980
- PMCID: PMC6395260
- DOI: 10.1016/j.jds.2015.12.001
Plasma rich in growth factors stimulates proliferation, migration, and gene expression associated with bone formation in human dental follicle cells
Abstract
Background/purpose: Plasma rich in growth factors (PRGFs), which is prepared from autologous blood from patients, has been reported with regards to bone regeneration for dental implants. Human dental follicle cells (hDFCs) have the capacity to commit to multiple cell types such as the osteoblastic lineage. The aim of this study is to evaluate the effects of PRGFs for mineralization in hDFCs.
Materials and methods: PRGFs was prepared from whole blood centrifuged at 460g for 8 minutes. hDFCs isolated from the dental follicle with collagenase/dispase were cultured with growth medium or osteogenic induction medium (OIM) containing PRGFs or fetal bovine serum. Concentrations of the growth factors were examined using an enzyme-linked immunosorbent assay kit. A cell migration assay was used for two-dimensional movement. Gene expressions were examined with real-time polymerase chain reaction using a DyNAmo SYBR Green quantitative polymerase chain reaction kit.
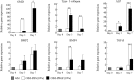
Results: The platelet concentration in PRGF Fraction 2 was 2.14-fold higher than in whole blood. White blood cells were not detected in PRGFs. Transforming growth factor-β levels were higher than insulin-like growth factor-1, platelet-derived growth factor-AB and -BB, and vascular endothelial growth factors in PRGF Fraction 2. Proliferation and migration by hDFCs increased in OIM supplemented with PRGFs in a dose-dependent manner and were higher in hDFCs cultured in OIM plus 10% PRGFs compared with OIM plus 10% fetal bovine serum. PRGFs upregulated the gene expression of type I collagen, osteomodulin, alkaline phosphatase, bone morphogenic protein-4, and transforming growth factor-β in hDFCs.
Conclusion: PRGFs may promote bone regeneration due to it including high levels of growth factors.
Keywords: PRGF; dental follicle cells; growth factors; osteogenic differentiation.
Figures



Similar articles
-
Bone morphogenetic protein 6 stimulates mineralization in human dental follicle cells without dexamethasone.Arch Oral Biol. 2013 Jun;58(6):690-8. doi: 10.1016/j.archoralbio.2012.10.018. Epub 2013 Jan 12. Arch Oral Biol. 2013. PMID: 23317773
-
Osteogenic differentiation and gene expression profile of human dental follicle cells induced by human dental pulp cells.J Mol Histol. 2015 Feb;46(1):93-106. doi: 10.1007/s10735-014-9604-1. Epub 2014 Dec 18. J Mol Histol. 2015. PMID: 25520056
-
Effect of inward rectifier potassium 2.1 channel on the osteogenic differentiation of human dental follicle cells and its mechanism.Hua Xi Kou Qiang Yi Xue Za Zhi. 2022 Mar 25;40(2):139-147. doi: 10.7518/hxkq.2022.02.003. Hua Xi Kou Qiang Yi Xue Za Zhi. 2022. PMID: 38597045 Free PMC article. Chinese, English.
-
[Effect of calcium on proliferation, migration and osteogenic differentiation of human dental follicle cells].Shanghai Kou Qiang Yi Xue. 2019 Dec;28(6):572-577. Shanghai Kou Qiang Yi Xue. 2019. PMID: 32346697 Chinese.
-
[Effect of hypoxia on the biological characteristics of human dental follicle cells].Hua Xi Kou Qiang Yi Xue Za Zhi. 2017 Jun 1;35(3):245-252. doi: 10.7518/hxkq.2017.03.004. Hua Xi Kou Qiang Yi Xue Za Zhi. 2017. PMID: 28675007 Free PMC article. Chinese.
Cited by
-
Multidifferentiation potential of dental-derived stem cells.World J Stem Cells. 2021 May 26;13(5):342-365. doi: 10.4252/wjsc.v13.i5.342. World J Stem Cells. 2021. PMID: 34136070 Free PMC article. Review.
-
The evolution of three generations of platelet concentrates products: a leap from classical formulations to the era of extracellular vesicles.Front Bioeng Biotechnol. 2025 Aug 7;13:1628565. doi: 10.3389/fbioe.2025.1628565. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40851809 Free PMC article. Review.
-
Clinical Outcomes of Root-Analogue Implants Restored with Single Crowns or Fixed Dental Prostheses: A Retrospective Case Series.J Clin Med. 2020 Jul 23;9(8):2346. doi: 10.3390/jcm9082346. J Clin Med. 2020. PMID: 32717843 Free PMC article.
-
Biologic Therapy in Chronic Pain Management: a Review of the Clinical Data and Future Investigations.Curr Pain Headache Rep. 2021 Mar 24;25(5):30. doi: 10.1007/s11916-021-00947-2. Curr Pain Headache Rep. 2021. PMID: 33761016 Review.
-
Dentin Growth after Direct Pulp Capping with the Different Fractions of Plasma Rich in Growth Factors (PRGF) vs. MTA: Experimental Study in Animal Model.J Clin Med. 2021 Jul 31;10(15):3432. doi: 10.3390/jcm10153432. J Clin Med. 2021. PMID: 34362215 Free PMC article.
References
-
- Fréchette J.P., Martineau I., Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84:434–439. - PubMed
-
- Marx R.E., Carlson E.R., Eichstaedt R.M., Schimmele S.R., Strauss J.E., Georgeff K.R. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. - PubMed
-
- Inchingolo F., Tatullo M., Marrelli M. Regenerative surgery performed with platelet-rich plasma used in sinus lift elevation before dental implant surgery: an useful aid in healing and regeneration of bone tissue. Eur Rev Med Pharmacol Sci. 2012;16:1222–1226. - PubMed
-
- Choi B.H., Im C.J., Huh J.Y., Suh J.J., Lee S.H. Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg. 2004;33:56–59. - PubMed
-
- Gerard D., Carlson E.R., Gotcher J.E., Jacobs M. Effects of platelet-rich plasma on the healing of autologous bone grafted mandibular defects in dogs. J Oral Maxillofac Surg. 2006;64:443–451. - PubMed
LinkOut - more resources
Full Text Sources

