Glioblastoma Stem- Like Cells, Metabolic Strategy to Kill a Challenging Target
- PMID: 30895167
- PMCID: PMC6415584
- DOI: 10.3389/fonc.2019.00118
Glioblastoma Stem- Like Cells, Metabolic Strategy to Kill a Challenging Target
Abstract
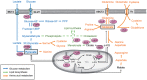
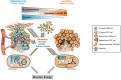
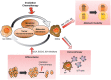
Over the years, substantial evidence has definitively confirmed the existence of cancer stem-like cells within tumors such as Glioblastoma (GBM). The importance of Glioblastoma stem-like cells (GSCs) in tumor progression and relapse clearly highlights that cancer eradication requires killing of GSCs that are intrinsically resistant to conventional therapies as well as eradication of the non-GSCs cells since GSCs emergence relies on a dynamic process. The past decade of research highlights that metabolism is a significant player in tumor progression and actually might orchestrate it. The growing interest in cancer metabolism reprogrammation can lead to innovative approaches exploiting metabolic vulnerabilities of cancer cells. These approaches are challenging since they require overcoming the compensatory and adaptive responses of GSCs. In this review, we will summarize the current knowledge on GSCs with a particular focus on their metabolic complexity. We will also discuss potential approaches targeting GSCs metabolism to potentially improve clinical care.
Keywords: Glioblastoma; cancer heterogeneity; cancer metabolism; cancer plasticity; cancer stem cells; tumor microenvironment.
Figures
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. (2009) 10:459–66. 10.1016/S1470-2045(09)70025-7 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

