No Significant Changes to Residual Viremia After Switch to Dolutegravir and Lamivudine in a Randomized Trial
- PMID: 30895201
- PMCID: PMC6419983
- DOI: 10.1093/ofid/ofz056
No Significant Changes to Residual Viremia After Switch to Dolutegravir and Lamivudine in a Randomized Trial
Abstract
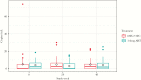
In the ASPIRE trial, antiretroviral therapy (ART) switch to dolutegravir plus lamivudine (DTG+3TC) was comparable to 3-drug ART in maintaining viral suppression by standard viral load assays. We used an ultrasensitive assay to assess whether this switch led to increased residual viremia. At entry, levels of residual viremia did not differ significantly between arms (DTG+3TC vs 3-drug ART: mean, 5.0 vs 4.2 HIV-1 RNA copies/mL; P = .64). After randomization, no significant between-group differences were found at either week 24 or 48. These results show no evidence for increased viral replication on DTG+3TC and support its further investigation as a dual ART strategy.
Keywords: ART simplification; ART switch; dolutegravir; lamivudine; residual viremia.
Figures
References
-
- Campillo-Gimenez L, Assoumou L, Valantin MA, et al. ROCnRAL ANRS 157 Study Group Switch to maraviroc/raltegravir dual therapy leads to an unfavorable immune profile with low-level HIV viremia. AIDS 2015; 29:853–6. - PubMed
-
- Blanco JL, Rojas J, Paredes R, et al. DOLAM Study Team Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother 2018; 73:1965–71. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

