Prevalence of Human Immunodeficiency Virus-1 Integrase Strand Transfer Inhibitor Resistance in British Columbia, Canada Between 2009 and 2016: A Longitudinal Analysis
- PMID: 30895202
- PMCID: PMC6419991
- DOI: 10.1093/ofid/ofz060
Prevalence of Human Immunodeficiency Virus-1 Integrase Strand Transfer Inhibitor Resistance in British Columbia, Canada Between 2009 and 2016: A Longitudinal Analysis
Abstract
Background: Integrase strand transfer inhibitors (INSTIs) are highly efficacious and well tolerated antiretrovirals with fewer adverse side-effects relative to other classes of antiretrovirals. The use of INSTIs raltegravir, elvitegravir, and dolutegravir has increased dramatically over recent years. However, there is limited information about the evolution and prevalence of INSTI resistance mutations in clinical human immunodeficiency virus populations.
Methods: Human immunodeficiency virus-1-positive individuals ≥19 years were included if they received ≥1 dispensed prescription of antiretroviral therapy (ART) in British Columbia between 2009 and 2016 (N = 9358). Physician-ordered drug resistance tests were analyzed and protease inhibitor (PI), reverse-transcriptase inhibitor (RT), and INSTI resistance were defined as having ≥1 sample with a combined, cumulative score ≥30 by Stanford HIV Drug Resistance Algorithm version 7.0.1.
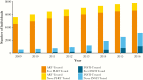
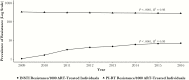
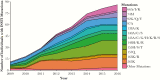
Results: Although most ART-treated individuals were tested for PI and RT resistance, INSTI resistance testing lagged behind the uptake of INSTIs among INSTI-treated individuals (11% in 2009; 34% in 2016). The prevalence of INSTI resistance was relatively low, but it increased from 1 to 7 per 1000 ART-treated individuals between 2009 and 2016 (P < .0001, R2 = 0.98). Integrase strand transfer inhibitor resistance mutations increased at integrase codons 66, 97, 140, 148, 155, and 263.
Conclusions: The prevalence of INSTI resistance remains low compared with PI and RT resistance in ART-treated populations but is expanding with increased INSTI use.
Keywords: HIV integrase strand transfer inhibitor; dolutegravir; drug resistance; elvitegravir; raltegravir.
Figures
References
-
- Lennox JL, DeJesus E, Lazzarin A, et al. . Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374:796–806. - PubMed
-
- Sax PE, DeJesus E, Mills A, et al. . Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379:2439–48. - PubMed
-
- World Health Organization. HIV drug resistance report 2017. Geneva: World Health Organization, 2017.
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents DHHS. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 4 June 2018.
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 4 June 2018.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

