Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers
- PMID: 30895304
- PMCID: PMC6594459
- DOI: 10.1093/annonc/mdz108
Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers
Abstract
Introduction: Immunotherapy is regarded as one of the major breakthroughs in cancer treatment. Despite its success, only a subset of patients responds-urging the quest for predictive biomarkers. We hypothesize that artificial intelligence (AI) algorithms can automatically quantify radiographic characteristics that are related to and may therefore act as noninvasive radiomic biomarkers for immunotherapy response.
Patients and methods: In this study, we analyzed 1055 primary and metastatic lesions from 203 patients with advanced melanoma and non-small-cell lung cancer (NSCLC) undergoing anti-PD1 therapy. We carried out an AI-based characterization of each lesion on the pretreatment contrast-enhanced CT imaging data to develop and validate a noninvasive machine learning biomarker capable of distinguishing between immunotherapy responding and nonresponding. To define the biological basis of the radiographic biomarker, we carried out gene set enrichment analysis in an independent dataset of 262 NSCLC patients.
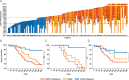
Results: The biomarker reached significant performance on NSCLC lesions (up to 0.83 AUC, P < 0.001) and borderline significant for melanoma lymph nodes (0.64 AUC, P = 0.05). Combining these lesion-wide predictions on a patient level, immunotherapy response could be predicted with an AUC of up to 0.76 for both cancer types (P < 0.001), resulting in a 1-year survival difference of 24% (P = 0.02). We found highly significant associations with pathways involved in mitosis, indicating a relationship between increased proliferative potential and preferential response to immunotherapy.
Conclusions: These results indicate that radiographic characteristics of lesions on standard-of-care imaging may function as noninvasive biomarkers for response to immunotherapy, and may show utility for improved patient stratification in both neoadjuvant and palliative settings.
Keywords: artificial intelligence; immunotherapy; machine learning; medical imaging; radiomics; response prediction.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures

Comment in
-
Radiomics to predict response to immunotherapy, bridging the gap from proof of concept to clinical applicability?Ann Oncol. 2019 Jun 1;30(6):879-881. doi: 10.1093/annonc/mdz150. Ann Oncol. 2019. PMID: 31124559 No abstract available.
-
A Machine Learning Algorithm for Predicting Therapeutic Response to Anti-PD1.Technol Cancer Res Treat. 2019 Jan 1;18:1533033819875766. doi: 10.1177/1533033819875766. Technol Cancer Res Treat. 2019. PMID: 31537168 Free PMC article. No abstract available.
References
-
- U.S. Food and Drug Administration. Drugs (KEYTRUDA Label). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125514s014l bl... (1 April 2019, date last accessed).
-
- U.S. Food and Drug Administration. Drugs (OPDIVO Label). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125554s055lbl.pdf (1 April 2019, date last accessed).
-
- Opdivo: EPAR—Product Information. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info... (1 April 2019, date last accessed).
-
- Keytruda: EPAR—Product Information. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info... (1 April 2019, date last accessed).
-
- Wolchok JD, Chiarion-Sileni V, Gonzalez R. et al. Efficacy and safety results from a phase III trial of nivolumab (NIVO) alone or combined with ipilimumab (IPI) versus IPI alone in treatment-naive patients (pts) with advanced melanoma (MEL) (CheckMate 067). JCO 2015; 33(Suppl 18): LBA1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

