A phase I study of gemcitabine + dasatinib (gd) or gemcitabine + dasatinib + cetuximab (GDC) in refractory solid tumors
- PMID: 30895346
- PMCID: PMC8040291
- DOI: 10.1007/s00280-019-03805-6
A phase I study of gemcitabine + dasatinib (gd) or gemcitabine + dasatinib + cetuximab (GDC) in refractory solid tumors
Abstract
Purpose: This study was conducted to define the maximum tolerated dose (MTD), recommended phase two dose (RPTD), and toxicities of gemcitabine + dasatinib (GD) and gemcitabine + dasatinib + cetuximab (GDC) in advanced solid tumor patients.
Methods: This study was a standard phase I 3 + 3 dose escalation study evaluating two combination regimens, GD and GDC. Patients with advanced solid tumors were enrolled in cohorts of 3-6 to either GD or GDC. Gemcitabine was dosed at 1000 mg/m2 weekly for 3 of 4 weeks, dasatinib was dosed in mg PO BID, and cetuximab was dosed at 250 mg/m2 weekly after a loading dose of cetuximab of 400 mg/m2. There were two dose levels for dasatinib: (1) gemcitabine + dasatinib 50 mg ± cetuximab, and (2) gemcitabine + dasatinib 70 mg ± cetuximab. Cycle length was 28 days. Standard cycle 1 dose-limiting toxicity (DLT) definitions were used. Eligible patients had advanced solid tumors, adequate organ and marrow function, and no co-morbidities that would increase the risk of toxicity. Serum, plasma, and skin biopsy biomarkers were obtained pre- and on-treatment.
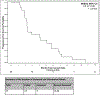
Results: Twenty-five patients were enrolled, including 21 with pancreatic adenocarcinoma. Three patients received prior gemcitabine. Twenty-one patients were evaluable for toxicity and 16 for response. Four DLTs were observed: Grade (Gr) 3 neutropenia (GDC1, n = 1), Gr 3 ALT (GD2, n = 2), and Gr 5 pneumonitis (GDC2, n = 1). Possible treatment-emergent adverse events (TEAEs) in later cycles included: Gr 3-4 neutropenia (n = 7), Gr 4 colitis (n = 1), Gr 3 bilirubin (n = 2), Gr 3 anemia (n = 2), Gr 3 thrombocytopenia (n = 2), Gr 3 edema/fluid retention (n = 1), and Gr 3 vomiting (n = 3). Six of 16 patients (3 of whom were gemcitabine-refractory) had stable disease (SD) as best response, median duration = 5 months (range 1-7). One gemcitabine-refractory patient had a partial response (PR). Median PFS was 2.9 months (95% CI 2.1, 5.8). Median OS was 5.8 months (95% CI 4.1, 11.8). Dermal wound biopsies demonstrated that dasatinib resulted in a decrease of total and phospho-Src levels, and cetuximab resulted in a decrease of EGFR and ERBB2 levels.
Conclusions: The MTD/RPTD of GD is gemcitabine 1000 mg/m2 weekly for 3 of 4 weeks and dasatinib 50 mg PO BID. The clinical activity of GD seen in this study was modest, and does not support its further investigation in pancreatic cancer.
Keywords: Cetuximab; Dasatinib; Gemcitabine; Pancreatic cancer; Phase I.
Conflict of interest statement
CONFLICT OF INTEREST
NBM is a clinical investigator on clinical trials supported by Incyte, ARMO, OncoMed, and Genentech/Roche. HU is a clinical investigator on clinical trials supported by Macrogenics, Merck, Genentech/Roche. ABN reports honoraria for consulting and advisory boards for Eli Lilly, Pfizer, and Kanghong Pharma. He has received grant support from Acceleron Pharma, Amgen, AstraZeneca/MedImmune, Eureka Therapeutics, Genentech, Leadiant Biosciences, MedPacto Inc, Novartis, Seattle Genetics, and Tracon Pharma. HH is employed by Genentech/Roche. The remaining authors have no conflicts of interest.
Figures


Similar articles
-
A phase 1 study of gemcitabine combined with dasatinib in patients with advanced solid tumors.Invest New Drugs. 2013 Aug;31(4):918-26. doi: 10.1007/s10637-012-9898-3. Epub 2012 Nov 20. Invest New Drugs. 2013. PMID: 23179336 Free PMC article. Clinical Trial.
-
Phase I study to evaluate multiple regimens of intravenous 5-fluorouracil administered in combination with weekly gemcitabine in patients with advanced solid tumors: a potential broadly active regimen for advanced solid tumor malignancies.Cancer. 2001 Sep 15;92(6):1567-76. doi: 10.1002/1097-0142(20010915)92:6<1567::aid-cncr1483>3.0.co;2-l. Cancer. 2001. PMID: 11745236 Clinical Trial.
-
A phase I trial of nab-paclitaxel, gemcitabine, and capecitabine for metastatic pancreatic cancer.Cancer Chemother Pharmacol. 2012 Dec;70(6):875-81. doi: 10.1007/s00280-012-1979-7. Epub 2012 Sep 28. Cancer Chemother Pharmacol. 2012. PMID: 23053263 Clinical Trial.
-
A phase I dose-escalation study of lenalidomide in combination with gemcitabine in patients with advanced pancreatic cancer.PLoS One. 2015 Apr 2;10(4):e0121197. doi: 10.1371/journal.pone.0121197. eCollection 2015. PLoS One. 2015. PMID: 25837499 Free PMC article. Clinical Trial.
-
Treatment of advanced pancreatic cancer: from gemcitabine single agent to combinations and targeted therapy.Cancer Treat Rev. 2009 Jun;35(4):335-9. doi: 10.1016/j.ctrv.2008.11.007. Epub 2009 Jan 7. Cancer Treat Rev. 2009. PMID: 19131170 Review.
Cited by
-
Opposite Effects of Src Family Kinases on YAP and ERK Activation in Pancreatic Cancer Cells: Implications for Targeted Therapy.Mol Cancer Ther. 2022 Nov 3;21(11):1652-1662. doi: 10.1158/1535-7163.MCT-21-0964. Mol Cancer Ther. 2022. PMID: 35999654 Free PMC article.
-
Phase I trial of Ganitumab plus Dasatinib to Cotarget the Insulin-Like Growth Factor 1 Receptor and Src Family Kinase YES in Rhabdomyosarcoma.Clin Cancer Res. 2023 Sep 1;29(17):3329-3339. doi: 10.1158/1078-0432.CCR-23-0709. Clin Cancer Res. 2023. PMID: 37398992 Free PMC article. Clinical Trial.
-
Novel biomarkers used for early diagnosis and tyrosine kinase inhibitors as targeted therapies in colorectal cancer.Front Pharmacol. 2023 Sep 1;14:1189799. doi: 10.3389/fphar.2023.1189799. eCollection 2023. Front Pharmacol. 2023. PMID: 37719843 Free PMC article. Review.
-
Targeting SRC Kinase Signaling in Pancreatic Cancer Stem Cells.Int J Mol Sci. 2020 Oct 9;21(20):7437. doi: 10.3390/ijms21207437. Int J Mol Sci. 2020. PMID: 33050159 Free PMC article.
-
Crosstalk between KRAS, SRC and YAP Signaling in Pancreatic Cancer: Interactions Leading to Aggressive Disease and Drug Resistance.Cancers (Basel). 2021 Oct 13;13(20):5126. doi: 10.3390/cancers13205126. Cancers (Basel). 2021. PMID: 34680275 Free PMC article. Review.
References
-
- Siegel RL et al. (2018) Cancer statistics, 2018. CA Cancer J Clin 68 (1), 7–30. - PubMed
-
- Ries LAG (2007) SEER Cancer Statistics Review, 1975–2005, National Cancer Institute. Bethesda, MD. . http://seer.cancer.gov/csr/1975_2005/, (accessed).
-
- Plunkett W et al. (1995) Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol 22 (4 Suppl 11), 3–10. - PubMed
-
- Burris HA 3rd et al. (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15 (6), 2403–13. - PubMed
-
- Flossmann-Kast BB et al. (1998) Src stimulates insulin-like growth factor I (IGF-I)-dependent cell proliferation by increasing IGF-I receptor number in human pancreatic carcinoma cells. Cancer Res 58 (16), 3551–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

