Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats
- PMID: 30895529
- PMCID: PMC6555783
- DOI: 10.1007/s10157-019-01727-4
Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats
Abstract
Background: Constipation is frequently observed in patients with chronic kidney disease (CKD). Lactulose is expected to improve the intestinal environment by stimulating bowel movements as a disaccharide laxative and prebiotic. We studied the effect of lactulose on renal function in adenine-induced CKD rats and monitored uremic toxins and gut microbiota.
Methods: Wistar/ST male rats (10-week-old) were fed 0.75% adenine-containing diet for 3 weeks to induce CKD. Then, they were divided into three groups and fed as follows: control, normal diet; and 3.0- and 7.5-Lac, 3.0% and 7.5% lactulose-containing diets, respectively, for 4 weeks. Normal diet group was fed normal diet for 7 weeks. The rats were observed for parameters including renal function, uremic toxins, and gut microbiota.
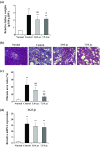
Results: The control group showed significantly higher serum creatinine (sCr) and blood urea nitrogen (BUN) 3 weeks after adenine feeding than at baseline, with a 8.5-fold increase in serum indoxyl sulfate (IS). After switching to 4 weeks of normal diet following adenine feeding, the sCr and BUN in control group remained high with a further increase in serum IS. In addition, tubulointerstitial fibrosis area was increased in control group. On the other hand, 3.0- and 7.5-Lac groups improved sCr and BUN levels, and suppressed tubulointerstitial fibrosis, suggesting preventing of CKD progression by lactulose. Lac groups also lowered level of serum IS and proportions of gut microbiota producing IS precursor.
Conclusion: Lactulose modifies gut microbiota and ameliorates CKD progression by suppressing uremic toxin production.
Keywords: CKD; Gut microbiota; Lactulose; Renal function; Uremic toxin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

