Nuclear orphan receptor NR2F6 confers cisplatin resistance in epithelial ovarian cancer cells by activating the Notch3 signaling pathway
- PMID: 30895619
- PMCID: PMC6767785
- DOI: 10.1002/ijc.32293
Nuclear orphan receptor NR2F6 confers cisplatin resistance in epithelial ovarian cancer cells by activating the Notch3 signaling pathway
Abstract
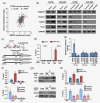
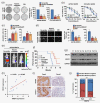
The primary challenge facing treatment of epithelial ovarian cancer (EOC) is the high frequency of chemoresistance, which severely impairs the quality of life and survival of patients with EOC. Our study aims to investigate the mechanisms by which upregulation of NR2F6 induces chemoresistance in EOC. The biological roles of NR2F6 in EOC chemoresistance were explored in vitro by Sphere, MTT and AnnexinV/PI assay, and in vivo using an ovarian cancer orthotopic transplantation model. Bioinformatics analysis, luciferase assay, CHIP and IP assays were performed to identify the mechanisms by which NR2F6 promotes chemoresistance in EOC. The expression of NR2F6 was significantly upregulated in chemoresistant EOC tissue, and NR2F6 expression was correlated with poorer overall survival. Moreover, overexpression of NR2F6 promotes the EOC cancer stem cell phenotype; conversely, knockdown of NR2F6 represses the EOC cancer stem cell phenotype and sensitizes EOC to cisplatin in vitro and in vivo. Our results further demonstrate that NR2F6 sustains activated Notch3 signaling, resulting in chemoresistance in EOC cells. Notably, NR2F6 acts as an informative biomarker to identify the population of EOC patients who are likely to experience a favorable objective response to gamma-secretase inhibitors (GSI), which inhibit Notch signaling. Therefore, concurrent inhibition of NR2F6 and treatment with GSI and cisplatin-based chemotherapy may be a novel therapeutic approach for NR2F6-overexpressing EOC. In summary, we have, for the first time, identified an important role for NR2F6 in EOC cisplatin resistance. Our study suggests that GSI may serve as a potential targeted treatment for patients with NR2F6-overexpressing EOC.
Keywords: NR2F6; Notch3 signaling pathway; cancer stem cells; chemoresistance; epithelial ovarian cancer.
© 2019 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Figures




References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. - PubMed
-
- Amate P, Huchon C, Dessapt AL, et al. Ovarian cancer: sites of recurrence. Int J Gynecol Cancer 2013;23:1590–6. - PubMed
-
- Tsai SY, Tsai MJ. Chick ovalbumin upstream promoter‐transcription factors (COUP‐TFs): coming of age. Endocr Rev 1997;18:229–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

