Mysm1 epigenetically regulates the immunomodulatory function of adipose-derived stem cells in part by targeting miR-150
- PMID: 30895711
- PMCID: PMC6484305
- DOI: 10.1111/jcmm.14281
Mysm1 epigenetically regulates the immunomodulatory function of adipose-derived stem cells in part by targeting miR-150
Abstract
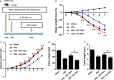
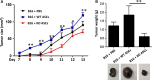
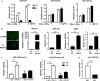
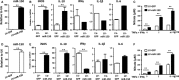
Adipose-derived stem cells (ASCs) are highly attractive for cell-based therapies in tissue repair and regeneration because they have multilineage differentiation capacity and are immunosuppressive. However, the detailed epigenetic mechanisms of their immunoregulatory capacity are not fully defined. In this study, we found that Mysm1 was induced in ASCs treated with inflammatory cytokines. Adipose-derived stem cells with Mysm1 knockdown exhibited attenuated immunosuppressive capacity, evidenced by less inhibition of T cell proliferation, more pro-inflammatory factor secretion and less nitric oxide (NO) production in vitro. Mysm1-deficient ASCs exacerbated inflammatory bowel diseases but inhibited tumour growth in vivo. Mysm1-deficient ASCs also showed depressed miR-150 expression. When transduced with Mysm1 overexpression lentivirus, ASCs exhibited enhanced miR-150 expression. Furthermore, Mysm1-deficient cells transduced with lentivirus containing miR-150 mimics produced less pro-inflammatory factors and more NO. Our study reveals a new role of Mysm1 in regulating the immunomodulatory activities of ASCs by targeting miR-150. These novel insights into the mechanisms through which ASCs regulate immune reactions may lead to better clinical utility of these cells.
Keywords: Mysm1; adipose-derived stem cells; miR-150; nitric oxide.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Deubiquitinase MYSM1 in the Hematopoietic System and beyond: A Current Review.Int J Mol Sci. 2020 Apr 24;21(8):3007. doi: 10.3390/ijms21083007. Int J Mol Sci. 2020. PMID: 32344625 Free PMC article. Review.
-
miR-129-5p Regulates the Immunomodulatory Functions of Adipose-Derived Stem Cells via Targeting Stat1 Signaling.Stem Cells Int. 2019 Oct 21;2019:2631024. doi: 10.1155/2019/2631024. eCollection 2019. Stem Cells Int. 2019. PMID: 31772586 Free PMC article.
-
Deubiquitinase MYSM1: An Important Tissue Development and Function Regulator.Int J Mol Sci. 2024 Dec 4;25(23):13051. doi: 10.3390/ijms252313051. Int J Mol Sci. 2024. PMID: 39684760 Free PMC article. Review.
-
MYSM1 inhibits human colorectal cancer tumorigenesis by activating miR-200 family members/CDH1 and blocking PI3K/AKT signaling.J Exp Clin Cancer Res. 2021 Oct 27;40(1):341. doi: 10.1186/s13046-021-02106-2. J Exp Clin Cancer Res. 2021. PMID: 34706761 Free PMC article.
-
MYSM1 Represses Innate Immunity and Autoimmunity through Suppressing the cGAS-STING Pathway.Cell Rep. 2020 Oct 20;33(3):108297. doi: 10.1016/j.celrep.2020.108297. Cell Rep. 2020. PMID: 33086059
Cited by
-
Deubiquitinase MYSM1 in the Hematopoietic System and beyond: A Current Review.Int J Mol Sci. 2020 Apr 24;21(8):3007. doi: 10.3390/ijms21083007. Int J Mol Sci. 2020. PMID: 32344625 Free PMC article. Review.
-
miR-129-5p Regulates the Immunomodulatory Functions of Adipose-Derived Stem Cells via Targeting Stat1 Signaling.Stem Cells Int. 2019 Oct 21;2019:2631024. doi: 10.1155/2019/2631024. eCollection 2019. Stem Cells Int. 2019. PMID: 31772586 Free PMC article.
-
Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application.Front Cell Dev Biol. 2020 Apr 17;8:236. doi: 10.3389/fcell.2020.00236. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32363193 Free PMC article. Review.
-
Deubiquitinase MYSM1: An Important Tissue Development and Function Regulator.Int J Mol Sci. 2024 Dec 4;25(23):13051. doi: 10.3390/ijms252313051. Int J Mol Sci. 2024. PMID: 39684760 Free PMC article. Review.
References
-
- Aksu AE, Rubin JP, Dudas JR, Marra KG. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose‐derived stem cells. Ann Plast Surg. 2008;60:306‐322. - PubMed
-
- Xie X, Wang Y, Zhao C, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP‐derived scaffold for cartilage regeneration. Biomaterials. 2012;33:7008‐7018. - PubMed
-
- Merceron C, Portron S, Masson M, et al. The effect of two‐ and three‐dimensional cell culture on the chondrogenic potential of human adipose‐derived mesenchymal stem cells after subcutaneous transplantation with an injectable hydrogel. Cell Transplant. 2011;20:1575‐1588. - PubMed
-
- Wosnitza M, Hemmrich K, Groger A, Gräber S, Pallua N. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation. 2007;75:12‐23. - PubMed
-
- Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue‐derived stromal vascular fraction and culture expanded adipose tissue‐derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641‐648. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

