Development and effects of tacrolimus-loaded nanoparticles on the inhibition of corneal allograft rejection
- PMID: 30895841
- PMCID: PMC6442111
- DOI: 10.1080/10717544.2019.1582728
Development and effects of tacrolimus-loaded nanoparticles on the inhibition of corneal allograft rejection
Abstract
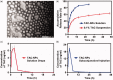
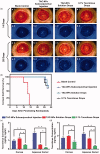
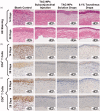
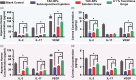
Tacrolimus has been widely applied to prevent organ rejection after transplantation. However, the conventional pharmaceutical formulation of tacrolimus limits its applications in ocular therapy due to its hydrophobicity and low corneal penetrability. We optimized tacrolimus-loaded methoxy poly (ethylene glycol-block-poly (d, l)-lactic-co-glycolic acid) nanoparticles (TAC-NPs) by simple and effective nanotechnology as a drug delivery system for corneal graft rejection to overcome these drawbacks. The prepared TAC-NPs were 82.9 ± 1.3 nm in size, and the drug loading and encapsulation efficiency were 8.01 ± 0.23% and 80.10 ± 2.33%. Furthermore, New Zealand rabbits were used to analyze the single-dose pharmacokinetics of the TAC-NPs using high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS). In rats with allogenic penetrating keratoplasty, the administration of TAC-NPs dispersion drops improved the TAC concentrations in the aqueous humor and cornea, consistent with a significantly higher effective inhibition of IL-2, IL-17, and VEGF expression compared with conventional 0.1% tacrolimus drops. Meanwhile, we also compared two different topical administration methods (including eye drop and subconjunctival injection) of TAC-NPs to maximize the sustained release characteristic of NPs. In summary, the small-sized TAC-NPs enhanced transcorneal permeation and absorption of TAC and more effectively inhibited corneal allograft rejection, which indicated that biodegradable polymeric nanomaterials-based drug delivery system had great potential for improving the clinical therapy efficacy of hydrophobic drugs.
Keywords: Tacrolimus; immune rejection; keratoplasty; mPEG-b-PLGA; nanoparticles.
Figures
References
-
- Barbu E, Verestiuc L, Nevell TG, Tsibouklis J (2006). Polymeric materials for ophthalmic drug delivery: trends and perspectives. J Mater Chem 16:3439–43.
-
- Chen X, Chen J, Li B, et al. (2017). PLGA-PEG-PLGA triblock copolymeric micelles as oral drug delivery system: in vitro drug release and in vivo pharmacokinetics assessment. J Colloid Interf Sci 490:542–52. - PubMed
-
- Dickey JB, Cassidy EM, Bouchard CS (1993). Periocular FK-506 delays allograft rejection in rat penetrating keratoplasty. Cornea 12:204–7. - PubMed
-
- Duncan R, Gaspar R (2011). Nanomedicine(s) under the microscope. Mol Pharm 8:2101–41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
