Role of spatial dispersion of repolarization in reentry around a functional core versus reentry around a fixed anatomical core
- PMID: 30896072
- PMCID: PMC6931426
- DOI: 10.1111/anec.12647
Role of spatial dispersion of repolarization in reentry around a functional core versus reentry around a fixed anatomical core
Abstract
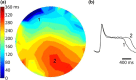
Introduction: Successful initiation of spiral wave reentry in the neonatal rat ventricular myocyte (NRVM) monolayer implicitly assumes the presence of spatial dispersion of repolarization (DR), which is difficult to quantify. We recently introduced a NRVM monolayer that utilizes anthopleurin-A to impart a prolonged plateau to the NRVM action potential. This was associated with a significant degree of spatial DR that lends itself to accurate quantification.
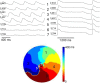
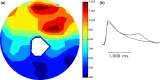
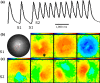
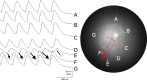
Methods and results: We utilized the monolayer and fluorescence optical mapping of intracellular calcium transients (FCai ) to systematically study and compare the contribution of spatial dispersion of the duration of FCai (as a surrogate of DR) to induction of spiral wave reentry around a functional core versus reentry around a fixed anatomical obstacle. We show that functional reentry could be initiated by a premature stimulus acting on a substrate of spatial DR resulting in a functional line of propagation block. Subsequent wave fronts circulated around a central core of functional obstacle created by sustained depolarization from the circulating wave front. Both initiation and termination of spiral wave reentry around an anatomical obstacle consistently required participation of a region of functional propagation block. This region was similarly based on spatial DR. Spontaneous termination of spiral wave reentry also resulted from block in the functional component of the circuit obstacle, usually preceded by beat-to-beat slowing of propagation.
Conclusions: The study demonstrates the critical contribution of DR to spiral wave reentry around a purely functional core as well as reentry around a fixed anatomical core.
Keywords: anthopleurin-A; dispersion of repolarization; neonatal rat ventricular myocyte monolayer; spiral wave reentry.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
There is no conflict of interest.
Figures



References
-
- Antezelevich, C. (2001). Heterogeneity of cellular repolarization in LQTS: The role of M cells. European Heart Journal Supplements, 3(Suppl. K), K2–K16. 10.1016/S1520-765X(01)90001-X - DOI
-
- Boutjdir, M. , Restivo, M. , Wei, Y. , Stergiopoulos, K. , & El‐Sherif, N. (1994). Early afterdepolarizations formation in cardiac myocytes: Analysis of phase plane patterns, action potential, and membrane currents. Journal of Cardiovascular Electrophysiology, 5(7), 609–620. 10.1111/j.1540-8167.1994.tb01302.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

