P34HB electrospun fibres promote bone regeneration in vivo
- PMID: 30896076
- PMCID: PMC6536444
- DOI: 10.1111/cpr.12601
P34HB electrospun fibres promote bone regeneration in vivo
Abstract
Objective: Bone tissue engineering was introduced in 1995 and provides a new way to reconstruct bone and repair bone defects. However, the design and fabrication of suitable bionic bone scaffolds are still challenging, and the ideal scaffolds in bone tissue engineering should have a three-dimensional porous network, good biocompatibility, excellent biodegradability and so on. The purpose of our research was to investigate whether a bioplasticpoly3-hydroxybutyrate4-hydroxybutyrate (P34HB) electrospun fibre scaffold is conducive to the repair of bone defects, and whether it is a potential scaffold for bone tissue engineering.
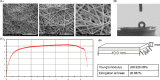
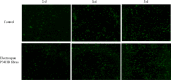
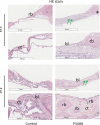
Materials and methods: The P34HB electrospun fibre scaffolds were prepared by electrospinning technology, and the surface morphology, hydrophilicity, mechanical properties and cytological behaviour of the scaffolds were tested. Furthermore, a calvarial defect model was created in rats, and through layer-by-layer paper-stacking technology, the P34HB electrospun fibre scaffolds were implanted into the calvarial defect area and their effect on bone repair was evaluated.
Results: The results showed that the P34HB electrospun fibre scaffolds are interwoven with several fibres and have good porosity, physical properties and chemical properties and can promote cell adhesion and proliferation with no cytotoxicity in vitro. In addition, the P34HB electrospun fibre scaffolds can promote the repair of calvarial defects in vivo.
Conclusions: These results demonstrated that the P34HB electrospun fibre scaffold has a three-dimensional porous network with good biocompatibility, excellent biosafety and ability for bone regeneration and repair; thus, the P34HB electrospun fibre scaffold is a potential scaffold for bone tissue engineering.
Keywords: P34HB; bone marrow mesenchymal stem cells; bone tissue engineering; calvarial defects; electrospinning.
© 2019 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.© 2019 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Figures



References
-
- Petite H, Viateau V, Bensaïd W, et al. Tissue‐engineered bone regeneration. Nat Biotechnol. 2000;18:959‐963. - PubMed
-
- Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenesis of biomimetic nano‐hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007;28:3338‐3348. - PubMed
-
- Stevens MM. Biomaterials for bone tissue engineering. Mater Today. 2008;11:18‐25.
-
- Bran GM, Stern‐Straeter J, Hörmann K, Riedel F, Goessler UR. Apoptosis in bone for tissue engineering. Arch Med Res. 2008;39:467‐482. - PubMed
-
- Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28:4240‐4250. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

