B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma
- PMID: 30896447
- PMCID: PMC6546468
- DOI: 10.1172/JCI126397
B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma
Abstract
Background: Chimeric antigen receptor (CAR) T cells are a promising therapy for hematologic malignancies. B-cell maturation antigen (BCMA) is a rational target in multiple myeloma (MM).
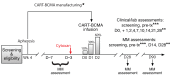
Methods: We conducted a phase I study of autologous T cells lentivirally-transduced with a fully-human, BCMA-specific CAR containing CD3ζ and 4-1BB signaling domains (CART-BCMA), in subjects with relapsed/refractory MM. Twenty-five subjects were treated in 3 cohorts: 1) 1-5 x 108 CART-BCMA cells alone; 2) Cyclophosphamide (Cy) 1.5 g/m2 + 1-5 x 107 CART-BCMA cells; and 3) Cy 1.5 g/m2 + 1-5 x 108 CART-BCMA cells. No pre-specified BCMA expression level was required.
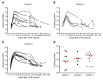
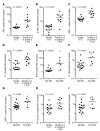
Results: CART-BCMA cells were manufactured and expanded in all subjects. Toxicities included cytokine release syndrome and neurotoxicity, which were grade 3-4 in 8 (32%) and 3 (12%) subjects, respectively, and reversible. One subject died at day 24 from candidemia and progressive myeloma, following treatment for severe CRS and encephalopathy. Responses (based on treated subjects) were seen in 4/9 (44%) in cohort 1, 1/5 (20%) in cohort 2, and 7/11 (64%) in cohort 3, including 5 partial, 5 very good partial, and 2 complete responses, 3 of which were ongoing at 11, 14, and 32 months. Decreased BCMA expression on residual MM cells was noted in responders; expression increased at progression in most. Responses and CART-BCMA expansion were associated with CD4:CD8 T cell ratio and frequency of CD45RO-CD27+CD8+ T cells in the pre-manufacturing leukapheresis product.
Conclusion: CART-BCMA infusions with or without lymphodepleting chemotherapy are clinically active in heavily-pretreated MM patients.
Trial registration: NCT02546167.
Funding: University of Pennsylvania-Novartis Alliance and NIH.
Keywords: Cancer immunotherapy; Clinical Trials; Oncology.
Conflict of interest statement
Figures



Comment in
-
BCMA CAR T cells: the winding path to success.J Clin Invest. 2019 Apr 29;129(6):2175-2177. doi: 10.1172/JCI128372. eCollection 2019 Apr 29. J Clin Invest. 2019. PMID: 31033482 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

