Inhibitory effects of petasin on human colon carcinoma cells mediated by inactivation of Akt/mTOR pathway
- PMID: 30896562
- PMCID: PMC6595872
- DOI: 10.1097/CM9.0000000000000199
Inhibitory effects of petasin on human colon carcinoma cells mediated by inactivation of Akt/mTOR pathway
Abstract
Background: Colorectal cancer is the third most common cancer worldwide and still lack of effective therapy so far. Petasin, a natural product found in plants of the genus Petasites, has been reported to possess anticancer activity. The present study aimed to investigate the anticolon cancer activity of petasin both in vitro and in vivo. The molecular mechanism of petasin was also further explored.
Methods: Caco-2, LoVo, SW-620, and HT-29 cell lines were used to detect the inhibitory effect of petasin on colon cancer proliferation. Cell viability was determined using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. Cell apoptosis was analyzed by flow cytometry. Hoechst 33258 staining was used to visualize morphological changes. Cell migration was assessed using a wound-healing migration assay, and cell invasion was investigated using Transwell chambers. Western blotting assays were employed to evaluate the expression levels of proteins in the protein kinase B/mammalian target of rapamycin (Akt/mTOR) signaling pathway. Finally, in vivo activity of petasin was evaluated using the SW-620 subcutaneous tumor model established in Balb/c nude mice. Twelve rats were randomly divided into control group and 10 mg/kg petasin group. The tumor volume was calculated every 7 days for 28 days. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay was performed to assess the apoptotic effect of petasin. Differences between two groups were assessed by analysis of independent-sample t tests.
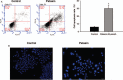
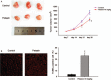
Results: Petasin significantly inhibited the proliferation of human colon carcinoma cell lines, induced apoptosis, and suppressed migration and invasion in SW-620 cells. Western blotting results showed that petasin decreased the phosphorylation of Akt (1.01 ± 0.16 vs. 0.74 ± 0.06, P = 0.042), mTOR (0.71 ± 0.12 vs. 0.32 ± 0.11, P = 0.013), and P70S6K (1.23 ± 0.21 vs. 0.85 ± 0.14, P = 0.008), elevated the expression of caspase-3 (0.41 ± 0.09 vs. 0.74 ± 0.12, P = 0.018) and caspase-9 (1.10 ± 0.27 vs. 1.98 ± 0.22, P = 0.009), decreased the Bcl-2 protein (2.75 ± 0.47 vs. 1.51 ± 0.36, P = 0.008), downregulated the expression of matrix metalloproteinase (MMP)-3 (1.51 ± 0.31 vs. 0.82 ± 0.11, P = 0.021) and MMP-9 (1.56 ± 0.32 vs. 0.94 ± 0.15, P = 0.039) in SW-620 cell. In vivo, 10 mg/kg petasin inhibited tumor growth in Balb/c nude mice (924.18 ± 101.23 vs. 577.67 ± 75.12 mm at day 28, P = 0.001) and induced apoptosis (3.6 ± 0.7% vs. 36.0 ± 4.9%, P = 0.001) in tumor tissues.
Conclusions: Petasin inhibits the proliferation of colon cancer SW-620 cells via inactivating the Akt/mTOR pathway. Our findings suggest petasin as a potential candidate for colon cancer therapy.
Figures



References
-
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014; 64:104–117. doi: 10.3322/caac.21220. - PubMed
-
- American Cancer Society: Global Cancer Facts & Figures. 2nd ed. Atlanta, GA: American Cancer Society; 2011.
-
- Banegas MP, Yabroff KR, O’Keeffe-Rosetti MC, Ritzwoller DP, Fishman PA, Salloum RG, et al. Medical care costs associated with cancer in integrated delivery systems. J Natl Compr Canc Netw 2018; 16:402–410. doi: 10.6004/jnccn.2017.7065. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

