Oral mucosal mesenchymal stem cell‑derived exosomes: A potential therapeutic target in oral premalignant lesions
- PMID: 30896790
- PMCID: PMC6438436
- DOI: 10.3892/ijo.2019.4756
Oral mucosal mesenchymal stem cell‑derived exosomes: A potential therapeutic target in oral premalignant lesions
Abstract
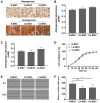
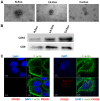
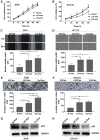
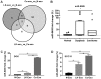
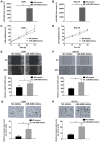
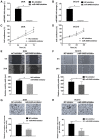
Emerging evidence indicates that mesenchymal stem cells (MSCs) serve an indispensable role in the tumor microenvironment. However, whether MSCs participate in the development of oral carcinogenesis remains unclear. The present study isolated MSCs from clinical tissues and investigated the differences of MSCs derived from normal oral mucosa (N‑MSC), oral leukoplakia with dysplasia (LK‑MSC) and oral carcinoma (Ca‑MSC). The results revealed that the LK‑MSCs exhibited reduced proliferation and migration, compared with the N‑MSCs and Ca‑MSCs. Furthermore, it was demonstrated that the exosomes secreted by LK‑MSCs have significant roles in promoting proliferation, migration and invasion in vitro, which was similar to the Ca‑MSC‑derived exosomes. The promoting effect was also demonstrated in a 3D coculture model. When the secretion of exosomes was blocked, the promoting effect of LK‑MSCs was reversed. Based on a microarray analysis of MSC‑derived exosomes, microRNA‑8485 (miR‑8485) was identified to be ectopically expressed. The exosomal miR‑8485 was capable of promoting the proliferation, migration and invasion of tumor cells. Therefore, the present study highlights the significance of MSC‑derived exosomes and exosomal miR‑8485 in premalignant lesions and carcinogenesis. Intervention with the secretion of MSC‑derived‑exosomes may be an innovative strategy to retard the carcinogenesis.
Figures

References
-
- Bouquot JE, Whitaker SB. Oral leukoplakia - rationale for diagnosis and prognosis of its clinical subtypes or 'phases'. Quintessence Int. 1994;25:133–140. - PubMed
-
- Kao SY, Mao L, Jian XC, Rajan G, Yu GY. Expert consensus on the detection and screening of oral cancer and precancer. Chin J Dent Res. 2015;18:79–83. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

