β‑estradiol alleviates hypertension‑ and concanavalin A‑mediated inflammatory responses via modulation of connexins in peripheral blood lymphocytes
- PMID: 30896818
- PMCID: PMC6471871
- DOI: 10.3892/mmr.2019.10037
β‑estradiol alleviates hypertension‑ and concanavalin A‑mediated inflammatory responses via modulation of connexins in peripheral blood lymphocytes
Abstract
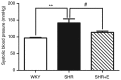
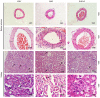
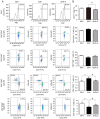
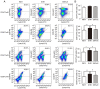
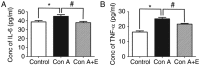
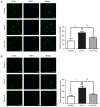
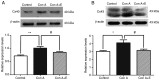
Gap junctions (GJs) formed by connexins (Cxs) in T lymphocytes have been reported to have important roles in the T lymphocyte‑driven inflammatory response and hypertension‑mediated inflammation. Estrogen has a protective effect on cardiovascular diseases, including hypertension and it attenuates excessive inflammatory responses in certain autoimmune diseases. However, the mechanisms involved in regulating the pro‑inflammatory response are complex and poorly understood. The current study investigated whether β‑estradiol suppresses hypertension and pro‑inflammatory stimuli‑mediated inflammatory responses by regulating Cxs and Cx‑mediated GJs in peripheral blood lymphocytes. Male, 16‑week‑old spontaneously hypertensive rats (SHR) and Wistar‑Kyoto rats (WKY) rats were randomly divided into the following three groups: WKY rats, vehicle (saline)‑treated SHRs, and β‑estradiol (20 µg/kg/day)‑treated SHRs. β‑estradiol was administered subcutaneously for 5 weeks. Hematoxylin and eosin staining was performed to evaluate target organ injury. Flow cytometry and ELISA were used to measure the populations of T lymphocyte subtypes in the peripheral blood, and expression of Cx40/Cx43 in T cell subtypes, and pro‑inflammation cytokines levels, respectively. ELISA, a dye transfer technique, immunofluorescence and immunoblotting were used to analyze the effect of β‑estradiol on pro‑inflammatory cytokine secretion, Cx‑mediated GJs and the expression of Cxs in concanavalin A (Con A)‑stimulated peripheral blood lymphocytes isolated from WKY rat. β‑estradiol significantly decreased blood pressure and inhibited hypertension‑induced target organ injury in SHRs. Additionally, β‑estradiol treatment significantly improved the immune homeostasis of SHRs, as demonstrated by the decreased percentage of cluster of differentiation (CD)4+/CD8+ T‑cell subset ratio, reduced serum levels of pro‑inflammatory cytokines and increased the percentage of CD4+CD25+ T cells. β‑estradiol also markedly reduced the expression of Cx40/Cx43 in T lymphocytes from SHRs. In vitro, β‑estradiol significantly suppressed the production of pro‑inflammatory cytokines, reduced communication via Cx‑mediated gap junctions and decreased the expression of Cx40/Cx43 in Con A‑stimulated lymphocytes. These results indicate that β‑estradiol attenuates inflammation and end organ damage in hypertension, which may be partially mediated via downregulated expression of Cxs and reduced function of Cx‑mediated GJ.
Figures
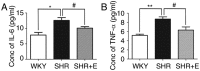

References
-
- Agita A, Alsagaff MT. Inflammation, immunity, and hypertension. Acta Med Indones. 2017;49:158–165. - PubMed
-
- Committee of Cardio-Cerebro-Vascular Diseases of Gerontological Society of China, Chinese College of Cardiovascular Physicians of Chinese Medical Doctor Association. Chinese expert consensus on the diagnosis and treatment of hypertension in the elderly (2017) Zhonghua Nei Ke Za Zhi. 2017;56:885–893. (In Chinese) - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

