Autophagy alleviates the decrease in proliferation of amyloid β1‑42‑treated bone marrow mesenchymal stem cells via the AKT/mTOR signaling pathway
- PMID: 30896831
- PMCID: PMC6471277
- DOI: 10.3892/mmr.2019.10069
Autophagy alleviates the decrease in proliferation of amyloid β1‑42‑treated bone marrow mesenchymal stem cells via the AKT/mTOR signaling pathway
Abstract
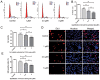
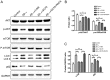
Alzheimer's disease (AD) and osteoporosis (OP) are 2 common progressive age‑associated diseases, primarily affecting the elderly worldwide. Accumulating evidence has demonstrated that patients with AD are more likely to suffer from bone mass loss and even OP, but whether it is a pathological feature of AD or secondary to motor dysfunction remains poorly understood. The present study aimed to investigate whether amyloid‑β1‑42 (Aβ1‑42), the typical pathological product of AD, exhibited a negative effect on the proliferation of bone marrow mesenchymal stem cells (BMSCs) and the role of autophagy. The proliferation of BMSCs was measured using a Cell Counting Kit‑8 assay, cell cycle analysis and 5‑ethynyl‑2'‑deoxyuridine (EdU) staining. The autophagy‑associated proteins microtubule‑associated proteins 1A/1B light chain 3B and sequestosome 1 (p62) were evaluated by western blot analysis and autophagosomes were detected by transmission electron microscopy and immunofluorescence. The activity of the protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway was measured using western blot analysis, and the autophagy inducer rapamycin (RAPA), inhibitor 3‑methyladenine (3‑MA) and the AKT activator SC79 were also used to investigate the role of AKT/mTOR signaling pathway and autophagy in the proliferation of BMSCs. The results suggested that the proliferation of BMSCs treated with Aβ1‑42 was inhibited, with the autophagy level increasing following treatment with Aβ1‑42 in a dose‑dependent manner, while the AKT/mTOR signaling pathway participated in the regulation of the autophagy level. Activation of autophagy using RAPA inhibited the decrease in proliferation of BMSCs, while suppression of autophagy by 3‑MA and activation of the AKT/mTOR signaling pathway increased the decrease in proliferation of BMSCs caused by Aβ1‑42. It was concluded that Aβ1‑42, as an external stimulus, suppressed the proliferation of BMSCs directly and that the AKT/mTOR signaling pathway participated in the regulation of the level of autophagy. Concomitantly, autophagy may serve as a resistance mechanism in inhibiting the decreased proliferation of BMSCs treated with Aβ1‑42.
Figures


Similar articles
-
Neuroprotective effects of bone marrow mesenchymal stem cells combined with mannitol on radiation-induced brain injury by regulating autophagy via the PI3K/AKT/mTOR signaling pathway.Brain Res Bull. 2025 May;224:111326. doi: 10.1016/j.brainresbull.2025.111326. Epub 2025 Mar 31. Brain Res Bull. 2025. PMID: 40174787
-
Knockdown of insulin-like growth factor 1 exerts a protective effect on hypoxic injury of aged BM-MSCs: role of autophagy.Stem Cell Res Ther. 2018 Oct 25;9(1):284. doi: 10.1186/s13287-018-1028-5. Stem Cell Res Ther. 2018. Retraction in: Stem Cell Res Ther. 2024 Nov 5;15(1):397. doi: 10.1186/s13287-024-04035-1. PMID: 30359321 Free PMC article. Retracted.
-
Enhancement of osteoporotic bone regeneration by strontium-substituted 45S5 bioglass via time-dependent modulation of autophagy and the Akt/mTOR signaling pathway.J Mater Chem B. 2021 Apr 28;9(16):3489-3501. doi: 10.1039/d0tb02991b. J Mater Chem B. 2021. PMID: 33690737
-
Research on the role and mechanism of the PI3K/Akt/mTOR signalling pathway in osteoporosis.Front Endocrinol (Lausanne). 2025 May 12;16:1541714. doi: 10.3389/fendo.2025.1541714. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40421249 Free PMC article. Review.
-
Targeting the mTOR-Autophagy Axis: Unveiling Therapeutic Potentials in Osteoporosis.Biomolecules. 2024 Nov 15;14(11):1452. doi: 10.3390/biom14111452. Biomolecules. 2024. PMID: 39595628 Free PMC article. Review.
Cited by
-
Recent advances of the mammalian target of rapamycin signaling in mesenchymal stem cells.Front Genet. 2022 Aug 30;13:970699. doi: 10.3389/fgene.2022.970699. eCollection 2022. Front Genet. 2022. PMID: 36110206 Free PMC article. Review.
-
Liquid-Liquid Phase Separation of Biomacromolecules and Its Roles in Metabolic Diseases.Cells. 2022 Sep 27;11(19):3023. doi: 10.3390/cells11193023. Cells. 2022. PMID: 36230986 Free PMC article. Review.
-
[Exendin-4 promotes autophagy to relieve lipid deposition in a NAFLD cell model by activating AKT/mTOR signaling pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2021 Jul 20;41(7):1073-1078. doi: 10.12122/j.issn.1673-4254.2021.07.16. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 34308859 Free PMC article. Chinese.
-
Growth factors-based beneficial effects of platelet lysate on umbilical cord-derived stem cells and their synergistic use in osteoarthritis treatment.Cell Death Dis. 2020 Oct 14;11(10):857. doi: 10.1038/s41419-020-03045-0. Cell Death Dis. 2020. PMID: 33057008 Free PMC article.
-
Programmed cell death in stem cell-based therapy: Mechanisms and clinical applications.World J Stem Cells. 2021 May 26;13(5):386-415. doi: 10.4252/wjsc.v13.i5.386. World J Stem Cells. 2021. PMID: 34136072 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

