Enhanced sensitivity of capture IgE‑ELISA based on a recombinant Der f 1/2 fusion protein for the detection of IgE antibodies targeting house dust mite allergens
- PMID: 30896856
- PMCID: PMC6472038
- DOI: 10.3892/mmr.2019.10050
Enhanced sensitivity of capture IgE‑ELISA based on a recombinant Der f 1/2 fusion protein for the detection of IgE antibodies targeting house dust mite allergens
Abstract
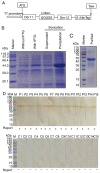
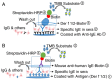
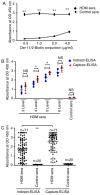
The detection of allergen‑specific immunoglobulin (Ig)E is an important method for the diagnosis of IgE‑mediated allergic diseases. The sensitivity of the indirect IgE‑ELISA method against allergen extracts is limited by interference from high IgG titers and low quantities of effectual allergen components in extracts. To overcome these limitations, a novel capture IgE‑ELISA based on a recombinant Der f 1/Der f 2 fusion protein (rDer f 1/2) was developed to enhance the sensitivity to IgEs that bind allergens from the house dust mite (HDM) species Dermatophagoides farina. pET28‑Der f 1/2 was constructed and expressed in Escherichia coli BL21 (DE3) pLysS. The purified fusion protein was evaluated by IgE western blotting, IgE dot blotting and indirect IgE‑ELISA. Capture‑ELISA was performed by coating wells with omalizumab and incubating in series with sera, biotinylated Der f 1/2, horseradish peroxidase‑conjugated streptavidin and 3,3,5,5‑tetramethylbenzidine. The relative sensitivities of indirect‑ELISA and capture‑ELISA for HDM allergen‑specific IgE binding were determined; sera from non‑allergic individuals were used as the control group. rDer f 1/2 was expressed in the form of inclusion bodies comprising refolded protein, which were then purified. It exhibited increased IgE‑specific binding (24/28, 85.8%) than rDer f 1 (21/28, 75.0%) or rDer f 2 (22/28, 78.6%) with HDM‑allergic sera. Furthermore, in a random sample of HDM‑allergic sera (n=71), capture‑ELISA (71/71, 100%) was more sensitive than indirect‑ELISA (68/71, 95.8%) for the detection of HDM‑specific IgEs (P<0.01), indicating that this novel method may be useful for the diagnosis of HDM allergy.
Figures


References
-
- Elkady A. Allergy to Dermatophagoides pteronyssinus (Der p1) and Dermatophagoides farina (Der f1) in patients with atopic asthma. Int J Sci Res. 2015;4:1896–1902.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

