LATS2 inhibits cell proliferation and metastasis through the Hippo signaling pathway in glioma
- PMID: 30896861
- PMCID: PMC6448087
- DOI: 10.3892/or.2019.7065
LATS2 inhibits cell proliferation and metastasis through the Hippo signaling pathway in glioma
Abstract
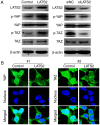
As a core kinase in the Hippo pathway, large tumor suppressor kinase 2 (LATS2) regulates cell proliferation, migration and invasion through numerous signaling pathways. However, its functions on cell proliferation, migration and invasion in glioma have yet to be elucidated. The present study revealed that LATS2 was downregulated in glioma tissues and cells, as determined by reverse transcription‑quantitative polymerase chain reaction and immunohistochemistry. In addition, Cell Counting Kit‑8, scratch wound healing and Transwell assays revealed that overexpression of LATS2 in U‑372 MG cells inhibited cell proliferation, migration and invasion. Furthermore, western blot analysis indicated that the expression levels of phosphorylated (p)‑yes‑associated protein and p‑tafazzin were increased in cells with LATS2 overexpression. These results indicated that LATS2 is a potential tumor suppressor, and downregulation of LATS2 in glioma may contribute to cancer progression.
Figures




Similar articles
-
LATS2 Inhibits Malignant Behaviors of Glioma Cells via Inactivating YAP.J Mol Neurosci. 2019 May;68(1):38-48. doi: 10.1007/s12031-019-1262-z. Epub 2019 Feb 15. J Mol Neurosci. 2019. PMID: 30771084
-
CMTM5 influences Hippo/YAP axis to promote ferroptosis in glioma through regulating WWP2-mediated LATS2 ubiquitination.Kaohsiung J Med Sci. 2024 Oct;40(10):890-902. doi: 10.1002/kjm2.12889. Epub 2024 Aug 21. Kaohsiung J Med Sci. 2024. PMID: 39166861 Free PMC article.
-
RASAL2 promotes tumor progression through LATS2/YAP1 axis of hippo signaling pathway in colorectal cancer.Mol Cancer. 2018 Jul 23;17(1):102. doi: 10.1186/s12943-018-0853-6. Mol Cancer. 2018. PMID: 30037330 Free PMC article.
-
Blockade of a novel MAP4K4-LATS2-SASH1-YAP1 cascade inhibits tumorigenesis and metastasis in luminal breast cancer.J Biol Chem. 2024 Jun;300(6):107309. doi: 10.1016/j.jbc.2024.107309. Epub 2024 Apr 22. J Biol Chem. 2024. PMID: 38657867 Free PMC article.
-
Downregulation of microRNA-224-3p Hampers Retinoblastoma Progression via Activation of the Hippo-YAP Signaling Pathway by Increasing LATS2.Invest Ophthalmol Vis Sci. 2020 Mar 9;61(3):32. doi: 10.1167/iovs.61.3.32. Invest Ophthalmol Vis Sci. 2020. PMID: 32186675 Free PMC article.
Cited by
-
TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation.Open Life Sci. 2021 Apr 8;16(1):323-335. doi: 10.1515/biol-2021-0039. eCollection 2021. Open Life Sci. 2021. PMID: 33889755 Free PMC article.
-
MicroRNA miR-4709-3p targets Large Tumor Suppressor Kinase 2 (LATS2) and induces obstructive renal fibrosis through Hippo signaling.Bioengineered. 2021 Dec;12(2):12357-12371. doi: 10.1080/21655979.2021.2002493. Bioengineered. 2021. PMID: 34931960 Free PMC article.
-
Recent Advances of the Hippo/YAP Signaling Pathway in Brain Development and Glioma.Cell Mol Neurobiol. 2020 May;40(4):495-510. doi: 10.1007/s10571-019-00762-9. Epub 2019 Nov 25. Cell Mol Neurobiol. 2020. PMID: 31768921 Free PMC article. Review.
-
Research Progress on the Regulation Mechanism of Key Signal Pathways Affecting the Prognosis of Glioma.Front Mol Neurosci. 2022 Jul 22;15:910543. doi: 10.3389/fnmol.2022.910543. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35935338 Free PMC article. Review.
-
CRISPR-cas9 screening identified lethal genes enriched in Hippo kinase pathway and of predictive significance in primary low-grade glioma.Mol Med. 2023 May 14;29(1):64. doi: 10.1186/s10020-023-00652-3. Mol Med. 2023. PMID: 37183261 Free PMC article.
References
-
- Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J, NABTT CNS Consortium Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. - DOI - PMC - PubMed
-
- Baker GJ, Yadav VN, Motsch S, Koschmann C, Calinescu AA, Mineharu Y, Camelopiragua S, Orringer D, Bannykh S, Nichols WS, et al. Mechanisms of glioma formation: Iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia. 2014;16:543–561. doi: 10.1016/j.neo.2014.06.003. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

