Sphingosine kinase and sphingosine-1-phosphate regulate epithelial cell architecture by the modulation of de novo sphingolipid synthesis
- PMID: 30897151
- PMCID: PMC6428330
- DOI: 10.1371/journal.pone.0213917
Sphingosine kinase and sphingosine-1-phosphate regulate epithelial cell architecture by the modulation of de novo sphingolipid synthesis
Abstract
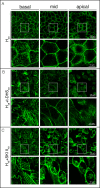
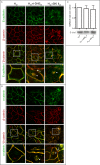
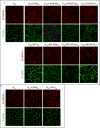
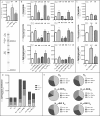
Sphingolipids regulate several aspects of cell behavior and it has been demonstrated that cells adjust their sphingolipid metabolism in response to metabolic needs. Particularly, sphingosine-1-phosphate (S1P), a final product of sphingolipid metabolism, is a potent bioactive lipid involved in the regulation of various cellular processes, including cell proliferation, cell migration, actin cytoskeletal reorganization and cell adhesion. In previous work in rat renal papillae, we showed that sphingosine kinase (SK) expression and S1P levels are developmentally regulated and control de novo sphingolipid synthesis. The aim of the present study was to evaluate the participation of SK/S1P pathway in the triggering of cell differentiation by external hypertonicity. We found that hypertonicity evoked a sharp decrease in SK expression, thus activating the de novo sphingolipid synthesis pathway. Furthermore, the inhibition of SK activity evoked a relaxation of cell-cell adherens junction (AJ) with accumulation of the AJ complex (E-cadherin/β-catenin/α-catenin) in the Golgi complex, preventing the acquisition of the differentiated cell phenotype. This phenotype alteration was a consequence of a sphingolipid misbalance with an increase in ceramide levels. Moreover, we found that SNAI1 and SNAI2 were located in the cell nucleus with impairment of cell differentiation induced by SK inhibition, a fact that is considered a biochemical marker of epithelial to mesenchymal transition. So, we suggest that the expression and activity of SK1, but not SK2, act as a control system, allowing epithelial cells to synchronize the various branches of sphingolipid metabolism for an adequate cell differentiation program.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- van Echten G, Birk R, Brenner-Weiss G, Schmidt RR, Sandhoff K. Modulation of sphingolipid biosynthesis in primary cultured neurons by long chain bases. J Biol Chem. United States; 1990;265: 9333–9339. - PubMed
-
- Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta. Netherlands; 2003;1632: 16–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

