Inflammatory monocytes are detrimental to the host immune response during acute infection with Cryptococcus neoformans
- PMID: 30897162
- PMCID: PMC6428256
- DOI: 10.1371/journal.ppat.1007627
Inflammatory monocytes are detrimental to the host immune response during acute infection with Cryptococcus neoformans
Abstract
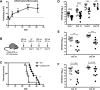
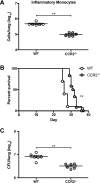
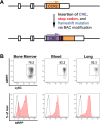
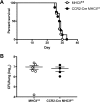
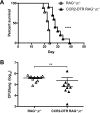
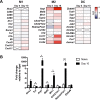
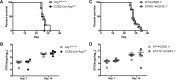
Cryptococcus neoformans is a leading cause of invasive fungal infections among immunocompromised patients. However, the cellular constituents of the innate immune response that promote clearance versus progression of infection upon respiratory acquisition of C. neoformans remain poorly defined. In this study, we found that during acute C. neoformans infection, CCR2+ Ly6Chi inflammatory monocytes (IM) rapidly infiltrate the lungs and mediate fungal trafficking to lung-draining lymph nodes. Interestingly, this influx of IM is detrimental to the host, since ablating IM or impairing their recruitment to the lungs improves murine survival and reduces fungal proliferation and dissemination. Using a novel conditional gene deletion strategy, we determined that MHC class II expression by IM did not mediate their deleterious impact on the host. Furthermore, although ablation of IM reduced the number of lymphocytes, innate lymphoid cells, and eosinophils in the lungs, the effects of IM were not dependent on these cells. We ascertained that IM in the lungs upregulated transcripts associated with alternatively activated (M2) macrophages in response to C. neoformans, consistent with the model that IM assume a cellular phenotype that is permissive for fungal growth. We also determined that conditional knockout of the prototypical M2 marker arginase 1 in IM and deletion of the M2-associated transcription factor STAT6 were not sufficient to reverse the harmful effects of IM. Overall, our findings indicate that C. neoformans can subvert the fungicidal potential of IM to enable the progression of infection through a mechanism that is not dependent on lymphocyte priming, eosinophil recruitment, or downstream M2 macrophage polarization pathways. These results give us new insight into the plasticity of IM function during fungal infections and the level of control that C. neoformans can exert on host immune responses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Heitman J, Kozel T. R., Kwon-Chung K. J., Perfect J. R. and Casadevall A. Cryptococcus: From Human Pathogen to Model Yeast 1 ed: ASM Press; 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

