Adapalene-loaded poly(ε-caprolactone) microparticles: Physicochemical characterization and in vitro penetration by photoacoustic spectroscopy
- PMID: 30897170
- PMCID: PMC6428289
- DOI: 10.1371/journal.pone.0213625
Adapalene-loaded poly(ε-caprolactone) microparticles: Physicochemical characterization and in vitro penetration by photoacoustic spectroscopy
Abstract
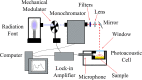
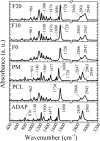
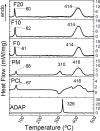
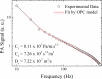
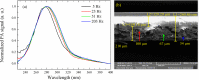
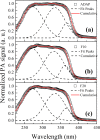
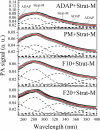
Adapalene (ADAP) is an important drug widely used in the topical treatment of acne. It is a third-generation retinoid and provides keratolytic, anti-inflammatory, and antiseborrhoic action. However, some topical adverse effects such as erythema, dryness, and scaling have been reported with its commercial formula. In this sense, the microencapsulation of this drug using polyesters can circumvent its topical side effects and can lead to the enhancement of drug delivery into sebaceous glands. The goal of this work was to obtain ADAP-loaded poly(ε-caprolactone) (PCL) microparticles prepared by a simple emulsion/solvent evaporation method. Formulations containing 10 and 20% of ADAP were successfully obtained and characterized by morphological, spectroscopic, and thermal studies. Microparticles presented encapsulation efficiency of ADAP above 98% and showed a smooth surface and spherical shape. Fourier transform infrared spectroscopy (FTIR) results presented no drug-polymer chemical bond, and a differential scanning calorimetry (DSC) technique showed a partial amorphization of the drug. ADAP permeation in the Strat-M membrane for transdermal diffusion testing was evaluated by photoacoustic spectroscopy (PAS) in the spectral region between 225 and 400 nm after 15 min and 3 h from the application of ADAP-loaded PCL formulations. PAS was successfully used for investigating the penetration of polymeric microparticles. In addition, microencapsulation decreased the in vitro transmembrane diffusion of ADAP.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Microencapsulation of cytarabine using poly(ethylene glycol)-poly(epsilon-caprolactone) diblock copolymers as surfactant agents.Drug Dev Ind Pharm. 2010 Apr;36(4):456-69. doi: 10.3109/03639040903261989. Drug Dev Ind Pharm. 2010. PMID: 19877831
-
Synthesis, characterizations, in vitro and in vivo evaluation of Etoricoxib-loaded Poly (Caprolactone) microparticles--a potential Intra-articular drug delivery system for the treatment of Osteoarthritis.J Biomater Sci Polym Ed. 2016;27(4):303-16. doi: 10.1080/09205063.2015.1125564. Epub 2016 Jan 11. J Biomater Sci Polym Ed. 2016. PMID: 26689653
-
Enhanced gastric tolerability and improved anti-obesity effect of capsaicinoids-loaded PCL microparticles.Mater Sci Eng C Mater Biol Appl. 2014 Jul 1;40:345-56. doi: 10.1016/j.msec.2014.03.049. Epub 2014 Mar 30. Mater Sci Eng C Mater Biol Appl. 2014. PMID: 24857502
-
PHBV/PCL microparticles for controlled release of resveratrol: physicochemical characterization, antioxidant potential, and effect on hemolysis of human erythrocytes.ScientificWorldJournal. 2012;2012:542937. doi: 10.1100/2012/542937. Epub 2012 May 1. ScientificWorldJournal. 2012. PMID: 22666135 Free PMC article.
-
The preparation and evaluation of poly(epsilon-caprolactone) microparticles containing both a lipophilic and a hydrophilic drug.J Control Release. 2000 Apr 3;65(3):429-38. doi: 10.1016/s0168-3659(99)00253-9. J Control Release. 2000. PMID: 10699300
Cited by
-
Recent Advances Regarding the Therapeutic Potential of Adapalene.Pharmaceuticals (Basel). 2020 Aug 28;13(9):217. doi: 10.3390/ph13090217. Pharmaceuticals (Basel). 2020. PMID: 32872149 Free PMC article. Review.
-
Targeted Topical Delivery of Retinoids in the Management of Acne Vulgaris: Current Formulations and Novel Delivery Systems.Pharmaceutics. 2019 Sep 24;11(10):490. doi: 10.3390/pharmaceutics11100490. Pharmaceutics. 2019. PMID: 31554188 Free PMC article. Review.
-
Design and Biocompatibility of Biodegradable Poly(octamethylene suberate) Nanoparticles to Treat Skin Diseases.Pharmaceutics. 2024 Jun 3;16(6):753. doi: 10.3390/pharmaceutics16060753. Pharmaceutics. 2024. PMID: 38931876 Free PMC article.
References
-
- Bernard BA. Adapalene, a New Chemical Entity with Retinoid Activity. Skin Pharmacol Physiol [Internet]. 1993;6(suppl 1)(Suppl. 1):61–9. Available from: https://www.karger.com/DOI/10.1159/000211165 - DOI - PubMed
-
- Osman-Ponchet H, Sevin K, Gaborit A, Wagner N, Poncet M. Fixed-Combination Gels of Adapalene and Benzoyl Peroxide Provide Optimal Percutaneous Absorption Compared to Monad Formulations of These Compounds: Results from Two In Vitro Studies. Dermatol Ther (Heidelb) [Internet]. 2017;7(1):123–31. Available from: 10.1007/s13555-016-0159-9 - DOI - PMC - PubMed
-
- Shroot B, Michel S, Galderma FC, Antipolis S. Pharmacology and chemistry of adapalene. J Am Acad Dermatol. 1997;36(6):S96–103. - PubMed
-
- Michel S, Jomard A, Demarchez M. Pharmacology of adapalene. Br J Dermatol. 1998;139:3–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

