Genetic and epigenetic profiling of the infertile male
- PMID: 30897172
- PMCID: PMC6428317
- DOI: 10.1371/journal.pone.0214275
Genetic and epigenetic profiling of the infertile male
Abstract
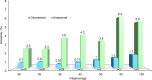
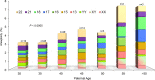
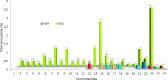
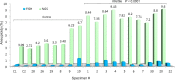
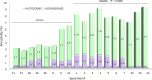
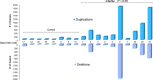
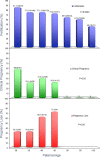
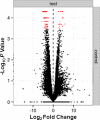
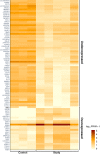
Evaluation of reproductive quality of spermatozoa by standard semen analysis is often inadequate to predict ART outcome. Men may be prone to meiotic error and have higher proportion of spermatozoa with fragmented chromatin, capable of affecting the conceptus' health. In men with unexplained infertility, supplementary tests may be pivotal to gain insight into the paternal contribution to the zygotic genome. A total of 113 consenting men were included in the study, with an additional 5 donor specimens used as control. Among study participants, 87 were screened for sperm aneuploidy by fluorescent in situ hybridization (FISH) and ranked according to their increasing age. A total of 18 men were assessed by whole exome sequencing and categorized according to their reproductive outcome as either fertile or infertile. Another set of men (n = 13) had their gene expression analyzed by RNA-seq and were profiled according to their reproductive capacity. FISH revealed that the average aneuploidy rate was highest for men over-55 age group (9.6%), while men >55 had the highest average disomy for chromosomes 17(1.2%) and 18(1.3%). ART results for the entire cohort comprised 157 cycles, stratified by paternal age. The youngest age group (25-30 years) had a fertilization rate of 87.7% which decreased to 46.0% in the >55 age group. Clinical pregnancy rate was highest in the 25-30yr group (80.0%) while no pregnancies were attained in the >55 age groups. Pregnancy loss was characterized by a steadily increasing trend, highest in the 51-55 age group (50.0%). NGS was performed on a cohort of patients classified as having recurrent pregnancy loss. This cohort was classified as the infertile group (n = 10) and was compared to a control group (n = 8) consisting of patients successfully treated by ART. Eight couples in 17 ICSI cycles achieved a clinical pregnancy rate of 82.4% while 10 infertile couples treated in 21 cycles achieved a pregnancy rate of 23.8%, all resulting in pregnancy loss. DNA-sequencing on spermatozoa from these patients yielded overall aneuploidy of 4.0% for fertile and 8.6% for the infertile group (P<0.00001). In the infertile cohort, we identified 17 genes with the highest mutation rate, engaged in key roles of gametogenesis, fertilization and embryo development. RNA-seq was performed on patients (n = 13) with normal semen analyses. Five men unable to attain a pregnancy after ART were categorized as the infertile group, while 8 men who successfully sustained a pregnancy were established as the fertile control. Analysis resulted in 86 differentially expressed genes (P<0.001). Of them, 24 genes were overexpressed and 62 were under-expressed in the infertile cohort. DNA repair genes (APLF, CYB5R4, ERCC4 and TNRFSF21) and apoptosis-modulating genes (MORC1, PIWIL1 and ZFAND6) were remarkably under-expressed (P<0.001). Sperm aneuploidy assessment supported by information on gene mutations may indicate subtle dysfunctions of the spermatozoon. Furthermore, by querying noncoding RNA we may gather knowledge on embryo developmental competence of spermatozoa, providing crucial information on the etiology of unexplained infertility of the infertile male.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Sabanegh ES. Male Infertility: Probloems and Solutions Eric A. Klein MD, editor. Cleveland, Ohio: Springer Science+Business Media, LLC; 2011.
-
- World Health Organization. WHO laboratory manual for the Examination and processing of human semen. 5th ed. Switzerland2010.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

