Amelioration of Huntington's disease phenotype in astrocytes derived from iPSC-derived neural progenitor cells of Huntington's disease monkeys
- PMID: 30897183
- PMCID: PMC6428250
- DOI: 10.1371/journal.pone.0214156
Amelioration of Huntington's disease phenotype in astrocytes derived from iPSC-derived neural progenitor cells of Huntington's disease monkeys
Abstract
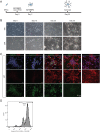
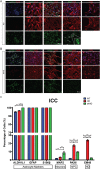
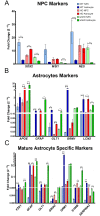
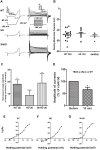
Huntington's disease (HD) is a devastating monogenic, dominant, hereditary, neurodegenerative disease. HD is caused by the expansion of CAG repeats in exon 1 of the huntingtin (HTT) gene, IT15, resulting in an expanded polyglutamine (polyQ) residue in the N-terminus of the HTT protein. HD is characterized by the accumulation of mutant HTT (mHTT) in neural and somatic cells. Progressive brain atrophy occurs initially in the striatum and extends to different brain regions with progressive decline in cognitive, behavioral and motor functions. Astrocytes are the most abundant cell type in the brain and play an essential role in neural development and maintaining homeostasis in the central nervous system (CNS). There is increasing evidence supporting the involvement of astrocytes in the development of neurodegenerative diseases such as Parkinson's disease (PD), Huntington's disease (HD), Alzheimer's disease (AD), and amyotrophic lateral sclerosis (ALS). We have generated neural progenitor cells (NPCs) from induced pluripotent stem cells (iPSCs) of transgenic HD monkeys as a model for studying HD pathogenesis. We have reported that NPCs can be differentiated in vitro into mature neural cells, such as neurons and glial cells, and are an excellent tool to study the pathogenesis of HD. To better understand the role of astrocytes in HD pathogenesis and discover new therapies to treat HD, we have developed an astrocyte differentiation protocol and evaluated the efficacy of RNAi to ameliorate HD phenotypes in astrocytes. The resultant astrocytes expressed canonical astrocyte-specific markers examined by immunostaining and real-time PCR. Flow cytometry (FACS) analysis showed that the majority of the differentiated NPCs (95.7%) were positive for an astrocyte specific marker, glial fibrillary acidic protein (GFAP). Functionalities of astrocytes were evaluated by glutamate uptake assay and electrophysiology. Expression of mHTT in differentiated astrocytes induced cytosolic mHTT aggregates and nuclear inclusions, suppressed the expression of SOD2 and PGC1, reduced ability to uptake glutamate, decreased 4-aminopyridine (4-AP) response, and shifted I/V plot measured by electrophysiology, which are consistent with previous reports on HD astrocytes and patient brain samples. However, expression of small-hairpin RNA against HTT (shHD) ameliorated and reversed aforementioned HD phenotypes in astrocytes. This represents a demonstration of a novel non-human primate (NHP) astrocyte model for studying HD pathogenesis and a platform for discovering novel HD treatments.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, et al. (2015) Huntington disease. Nature Reviews Disease Primers: 15005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

