The vexing complexity of the amyloidogenic pathway
- PMID: 30897251
- PMCID: PMC6566549
- DOI: 10.1002/pro.3606
The vexing complexity of the amyloidogenic pathway
Abstract
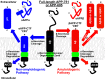
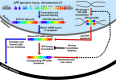
The role of the amyloidogenic pathway in the etiology of Alzheimer's disease (AD), particularly the common sporadic late onset forms of the disease, is controversial. To some degree, this is a consequence of the failure of drug and therapeutic antibody trials based either on targeting the proteases in this pathway or its amyloid end products. Here, we explore the formidable complexity of the biochemistry and cell biology associated with this pathway. For example, we review evidence that the immediate precursor of amyloid-β, the C99 domain of the amyloid precursor protein (APP), may itself be toxic. We also review important new results that appear to finally establish a direct genetic link between mutations in APP and the sporadic forms of AD. Based on the complexity of amyloidogenesis, it seems possible that a major contributor to the failure of related drug trials is that we have an incomplete understanding of this pathway and how it is linked to Alzheimer's pathogenesis. If so, this highlights a need for further characterization of this pathway, not its abandonment.
Keywords: APP-CTF; Alzheimer's disease; Aβ; C99; EOAD; FAD; LOAD; SAD; amyloid; amyloid precursor protein; amyloidogenesis; amyloidogenic; drug trials; etiology; gencDNA; gene; mutants; mutations; neurodegeneration; pathogenesis; plaques; presenilin; therapeutics; variants; variations; α-secretase; β-secretase; γ-secretase.
© 2019 The Protein Society.
Conflict of interest statement
The authors declare that they have no conflicts of interest related to this work.
Figures



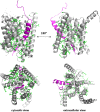
References
-
- Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N (2018) NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 14:535–562. - PMC - PubMed
-
- Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ (2013) Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12:207–216. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

