Pharmacokinetic Properties of Ibuprofen (IBU) From the Fixed-Dose Combination IBU/Caffeine (400/100 mg; FDC) in Comparison With 400 mg IBU as Acid or Lysinate Under Fasted and Fed Conditions-Data From 2 Single-Center, Single-Dose, Randomized Crossover Studies in Healthy Volunteers
- PMID: 30897305
- PMCID: PMC6767367
- DOI: 10.1002/cpdd.672
Pharmacokinetic Properties of Ibuprofen (IBU) From the Fixed-Dose Combination IBU/Caffeine (400/100 mg; FDC) in Comparison With 400 mg IBU as Acid or Lysinate Under Fasted and Fed Conditions-Data From 2 Single-Center, Single-Dose, Randomized Crossover Studies in Healthy Volunteers
Abstract
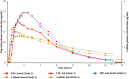
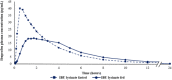
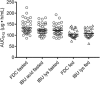
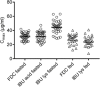
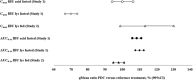
Rapid onset of analgesic action is linked with rapid absorption of analgesics (high maximum concentration [Cmax ] and short time to maximum concentration [tmax ]). After overnight fasting, ibuprofen lysinate reaches higher peak plasma levels (Cmax ) earlier than ibuprofen acid (tmax ) with comparable exposure (area under the plasma concentration-time curve [AUC]); however, subjects usually take ibuprofen with or within a short time of a meal. Therefore, pharmacokinetic (PK) studies under fed conditions may better characterize properties under real-life conditions. We investigated a new fixed-dose combination (FDC) of ibuprofen acid 400 mg and caffeine 100 mg in 2 single-dose, randomized, crossover PK studies in healthy subjects (both N = 36). The FDC was compared with ibuprofen 400 mg as acid and as lysinate after an overnight fast in Study 1, and with ibuprofen lysinate after a meal in Study 2. After fasting, results for ibuprofen in the FDC were comparable with those from ibuprofen acid alone. Caffeine did not affect the Cmax , tmax , and AUC. As expected, a higher Cmax and shorter tmax were observed with ibuprofen lysinates vs the FDC. Compared with administration after fasting, Cmax and tmax for ibuprofen lysinate administered postprandially were markedly different, while with FDC, these parameters were less sensitive to food intake. Taken after a meal, ibuprofen in the FDC reached tmax earlier than ibuprofen lysinate (median 1.25 vs 1.63 hours), and Cmax was approximately 13% higher, with comparable AUC, suggesting that the profile of ibuprofen was in favor of the FDC compared with ibuprofen lysinate. Thus, under real-life conditions, ibuprofen lysinate had no PK advantage over the FDC. All preparations were well tolerated.
Keywords: bioavailability; caffeine; fed; food effect; ibuprofen; lysinate.
© 2019 The Authors. Clinical Pharmacology in Drug Development Published by Wiley Periodicals, Inc. on behalf of The American College of Clinical Pharmacology.
Conflict of interest statement
T.W., R.L., and T.M. are employees of Sanofi‐Aventis Deutschland GmbH. C.S. is an employee of Boehringer Ingelheim Pharma GmbH & Co. KG. The consumer health care business was transferred to Sanofi effective on January 1, 2017.
Figures
References
-
- Rainsford KD. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology. 2009;17:275‐342. - PubMed
-
- Moore RA, Derry S, Straube S, Ireson‐Paine J, Wiffen PJ. Faster, higher, stronger? Evidence for formulation and efficacy for ibuprofen in acute pain. Pain. 2014;155:14‐21. - PubMed
-
- Derry CJ, Derry S, Moore RA. Caffeine as an analgesic adjuvant for acute pain in adults (review). Cochrane Database Syst Rev. 2012;14:CD009281. - PubMed
-
- Davies NM. Clinical pharmacokinetics of ibuprofen: the first 30 years. Clin Pharmacokinet. 1998;34:101‐154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

