Systematic Evaluation of Genetic and Environmental Factors Affecting Performance of Translational Riboswitches
- PMID: 30897329
- PMCID: PMC6492952
- DOI: 10.1021/acssynbio.9b00017
Systematic Evaluation of Genetic and Environmental Factors Affecting Performance of Translational Riboswitches
Abstract
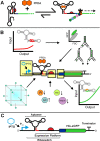
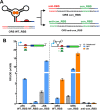
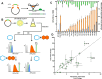
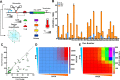
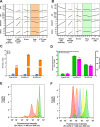
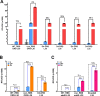
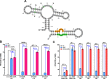
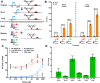
Since their discovery, riboswitches have been attractive tools for the user-controlled regulation of gene expression in bacterial systems. Riboswitches facilitate small molecule mediated fine-tuning of protein expression, making these tools of great use to the synthetic biology community. However, the use of riboswitches is often restricted due to context dependent performance and limited dynamic range. Here, we report the drastic improvement of a previously developed orthogonal riboswitch achieved through in vivo functional selection and optimization of flanking coding and noncoding sequences. The behavior of the derived riboswitches was mapped under a wide array of growth and induction conditions, using a structured Design of Experiments approach. This approach successfully improved the maximal protein expression levels 8.2-fold relative to the original riboswitches, and the dynamic range was improved to afford riboswitch dependent control of 80-fold. The optimized orthogonal riboswitch was then integrated downstream of four endogenous stress promoters, responsive to phosphate starvation, hyperosmotic stress, redox stress, and carbon starvation. These responsive stress promoter-riboswitch devices were demonstrated to allow for tuning of protein expression up to ∼650-fold in response to both environmental and cellular stress responses and riboswitch dependent attenuation. We envisage that these riboswitch stress responsive devices will be useful tools for the construction of advanced genetic circuits, bioprocessing, and protein expression.
Keywords: Design of Experiments; FACS; cellular stress; riboswitch; robustness; synthetic biology.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Systematic Comparison and Rational Design of Theophylline Riboswitches for Effective Gene Repression.Microbiol Spectr. 2023 Feb 14;11(1):e0275222. doi: 10.1128/spectrum.02752-22. Epub 2023 Jan 23. Microbiol Spectr. 2023. PMID: 36688639 Free PMC article.
-
Conditional control of gene expression by synthetic riboswitches in Streptomyces coelicolor.Methods Enzymol. 2015;550:283-99. doi: 10.1016/bs.mie.2014.10.036. Epub 2014 Dec 26. Methods Enzymol. 2015. PMID: 25605391
-
A Theophylline-Responsive Riboswitch Regulates Expression of Nuclear-Encoded Genes.Plant Physiol. 2020 Jan;182(1):123-135. doi: 10.1104/pp.19.00625. Epub 2019 Nov 8. Plant Physiol. 2020. PMID: 31704721 Free PMC article.
-
Riboswitch distribution, structure, and function in bacteria.Gene. 2019 Aug 5;708:38-48. doi: 10.1016/j.gene.2019.05.036. Epub 2019 May 22. Gene. 2019. PMID: 31128223 Review.
-
Unexpected versatility in bacterial riboswitches.Trends Genet. 2015 Mar;31(3):150-6. doi: 10.1016/j.tig.2015.01.005. Epub 2015 Feb 21. Trends Genet. 2015. PMID: 25708284 Review.
Cited by
-
Riboswitches for Controlled Expression of Therapeutic Transgenes Delivered by Adeno-Associated Viral Vectors.Pharmaceuticals (Basel). 2021 Jun 10;14(6):554. doi: 10.3390/ph14060554. Pharmaceuticals (Basel). 2021. PMID: 34200913 Free PMC article. Review.
-
Aptamer-Array-Guided Protein Assembly Enhances Synthetic mRNA Switch Performance.Angew Chem Int Ed Engl. 2022 Aug 22;61(34):e202207319. doi: 10.1002/anie.202207319. Epub 2022 Jul 13. Angew Chem Int Ed Engl. 2022. PMID: 35703374 Free PMC article.
-
Assembly of functional microbial ecosystems: from molecular circuits to communities.FEMS Microbiol Rev. 2024 Nov 23;48(6):fuae026. doi: 10.1093/femsre/fuae026. FEMS Microbiol Rev. 2024. PMID: 39496507 Free PMC article. Review.
-
Data-driven learning of structure augments quantitative prediction of biological responses.PLoS Comput Biol. 2024 Jun 3;20(6):e1012185. doi: 10.1371/journal.pcbi.1012185. eCollection 2024 Jun. PLoS Comput Biol. 2024. PMID: 38829926 Free PMC article.
-
Regulatory RNAs in Bacillus subtilis: A review on regulatory mechanism and applications in synthetic biology.Synth Syst Biotechnol. 2024 Feb 10;9(2):223-233. doi: 10.1016/j.synbio.2024.01.013. eCollection 2024 Jun. Synth Syst Biotechnol. 2024. PMID: 38385150 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

