Lack of galectin-1 exacerbates chronic hepatitis, liver fibrosis, and carcinogenesis in murine hepatocellular carcinoma model
- PMID: 30897344
- PMCID: PMC9292271
- DOI: 10.1096/fj.201900017R
Lack of galectin-1 exacerbates chronic hepatitis, liver fibrosis, and carcinogenesis in murine hepatocellular carcinoma model
Abstract
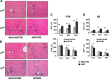
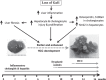
Chronic liver inflammation (CLI) is a risk factor for development of hepatocellular carcinoma (HCC). Galectin-1 (Gal1) is involved in the regulation of inflammation, angiogenesis, and tumorigenesis, exhibiting multiple anti-inflammatory and protumorigenic activities. We aimed to explore its regulatory role in CLI and HCC progression using an established model of CLI-mediated HCC development, Abcb4 [multidrug-resistance 2 (Mdr2)]-knockout (KO) mice, which express high levels of Gal1 in the liver. We generated double-KO (dKO) Gal1-KO/Mdr2-KO mice on C57BL/6 and FVB/N genetic backgrounds and compared HCC development in the generated strains with their parental Mdr2-KO strains. Loss of Gal1 increased liver injury, inflammation, fibrosis, and ductular reaction in dKO mice of both strains starting from an early age. Aged dKO mutants displayed earlier hepatocarcinogenesis and increased tumor size compared with control Mdr2-KO mice. We found that osteopontin, a well-known modulator of HCC development, and oncogenic proteins Ntrk2 (TrkB) and S100A4 were overexpressed in dKO compared with Mdr2-KO livers. Our results demonstrate that in Mdr2-KO mice, a model of CLI-mediated HCC, Gal1-mediated protection from hepatitis, liver fibrosis, and HCC initiation dominates over its known procarcinogenic activities at later stages of HCC development. These findings suggest that anti-Gal1 treatments may not be applicable at all stages of CLI-mediated HCC.-Potikha, T., Pappo, O., Mizrahi, L., Olam, D., Maller, S. M., Rabinovich, G. A., Galun, E., Goldenberg, D. S. Lack of galectin-1 exacerbates chronic hepatitis, liver fibrosis, and carcinogenesis in murine hepatocellular carcinoma model.
Keywords: HCC; Mdr2; Ntrk2; S100a4; Spp1.
Figures







References
-
- Balkwill, F. R. , and Mantovani, A. (2012) Cancer-related inflammation: common themes and therapeutic opportunities. Semin. Cancer Biol. 22, 33–40 - PubMed
-
- Mauad, T. H. , van Nieuwkerk, C. M. , Dingemans, K. P. , Smit, J. J. , Schinkel, A. H. , Notenboom, R. G. , van den Bergh Weerman, M. A. , Verkruisen, R. P. , Groen, A. K. , Oude Elferink, R. P. J. , van der Valk, M. A. , Borst, P. , and Offerhaus, G. J. A. (1994) Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am. J. Pathol. 145, 1237–1245 - PMC - PubMed
-
- Katzenellenbogen, M. , Pappo, O. , Barash, H. , Klopstock, N. , Mizrahi, L. , Olam, D. , Jacob-Hirsch, J. , Amariglio, N. , Rechavi, G. , Mitchell, L. A. , Kohen, R. , Domany, E. , Galun, E. , and Goldenberg, D. (2006) Multiple adaptive mechanisms to chronic liver disease revealed at early stages of liver carcinogenesis in the Mdr2-knockout mice. Cancer Res. 66, 4001–4010 - PubMed
-
- Sundblad, V. , Morosi, L. G. , Geffner, J. R. , and Rabinovich, G. A. (2017) Galectin-1: a Jack-of-all-trades in the resolution of acute and chronic inflammation. J. Immunol. 199, 3721–3730 - PubMed
-
- Camby, I. , Le Mercier, M. , Lefranc, F. , and Kiss, R. (2006) Galectin-1: a small protein with major functions. Glycobiobgy 16, 137R–157R - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

