BOLD-fMRI activity informed by network variation of scalp EEG in juvenile myoclonic epilepsy
- PMID: 30897433
- PMCID: PMC6425117
- DOI: 10.1016/j.nicl.2019.101759
BOLD-fMRI activity informed by network variation of scalp EEG in juvenile myoclonic epilepsy
Abstract
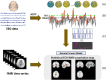
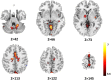
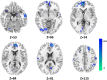
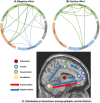
Epilepsy is marked by hypersynchronous bursts of neuronal activity, and seizures can propagate variably to any and all areas, leading to brain network dynamic organization. However, the relationship between the network characteristics of scalp EEG and blood oxygenation level-dependent (BOLD) responses in epilepsy patients is still not well known. In this study, simultaneous EEG and fMRI data were acquired in 18 juvenile myoclonic epilepsy (JME) patients. Then, the adapted directed transfer function (ADTF) values between EEG electrodes were calculated to define the time-varying network. The variation of network information flow within sliding windows was used as a temporal regressor in fMRI analysis to predict the BOLD response. To investigate the EEG-dependent functional coupling among the responding regions, modulatory interactions were analyzed for network variation of scalp EEG and BOLD time courses. The results showed that BOLD activations associated with high network variation were mainly located in the thalamus, cerebellum, precuneus, inferior temporal lobe and sensorimotor-related areas, including the middle cingulate cortex (MCC), supplemental motor area (SMA), and paracentral lobule. BOLD deactivations associated with medium network variation were found in the frontal, parietal, and occipital areas. In addition, modulatory interaction analysis demonstrated predominantly directional negative modulation effects among the thalamus, cerebellum, frontal and sensorimotor-related areas. This study described a novel method to link BOLD response with simultaneous functional network organization of scalp EEG. These findings suggested the validity of predicting epileptic activity using functional connectivity variation between electrodes. The functional coupling among the thalamus, frontal regions, cerebellum and sensorimotor-related regions may be characteristically involved in epilepsy generation and propagation, which provides new insight into the pathophysiological mechanisms and intervene targets for JME.
Keywords: Functional coupling; Juvenile myoclonic epilepsy; Modulatory interaction; Network variation; Simultaneous EEG and fMRI.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Abreu R., Leal A., da Silva F.L., Figueiredo P. EEG synchronization measures predict epilepsy-related BOLD-fMRI fluctuations better than commonly used univariate metrics. Clin. Neurophysiol. 2018;129(3):618–635. - PubMed
-
- Aghakhani Y., Bagshaw A.P., Benar C.G., Hawco C., Andermann F., Dubeau F. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127:1127–1144. - PubMed
-
- Allen P.J., Josephs O., Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage. 2000;12(2):230–239. - PubMed
-
- Arnold M., Miltner W.H., Witte H., Bauer R., Braun C. Adaptive AR modeling of nonstationary time series by means of Kalman filtering. IEEE Trans. Biomed. Eng. 1998;45(5):553–562. - PubMed
-
- Baykan B., Wolf P. Juvenile myoclonic epilepsy as a spectrum disorder: a focused review. Seizure. 2017;49:36–41. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

