Hyaluronan Derived From the Limbus is a Key Regulator of Corneal Lymphangiogenesis
- PMID: 30897620
- PMCID: PMC6432804
- DOI: 10.1167/iovs.18-25920
Hyaluronan Derived From the Limbus is a Key Regulator of Corneal Lymphangiogenesis
Abstract
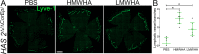
Purpose: We recently reported that the glycosaminoglycan hyaluronan (HA), which promotes inflammatory angiogenesis in other vascular beds, is an abundant component of the limbal extracellular matrix. Consequently, we have explored the possibility that HA contributes to lymphangiogenesis in the inflamed cornea.
Methods: To study the role of HA on lymphangiogenesis, we used mice lacking the hyaluronan synthases and injury models that induce lymphangiogenesis.
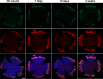
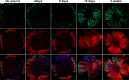
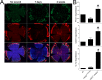
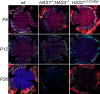
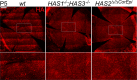
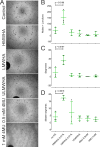
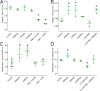
Results: Here we report that HA regulates corneal lymphangiogenesis, both during post-natal development and in response to adult corneal injury. Furthermore, we show that injury to the cornea by alkali burn upregulates both HA production and lymphangiogenesis and that these processes are ablated in HA synthase 2 deficient mice.
Conclusion: These findings raise the possibility that therapeutic blockade of HA-mediated lymphangiogenesis might prevent the corneal scarring and rejection that frequently results from corneal transplantation.
Figures

References
-
- Brown P. Lymphatic system: unlocking the drains. Nature. 2005;436:456–458. - PubMed
-
- Hos D, Bachmann B, Bock F, Onderka J, Cursiefen C. Age-related changes in murine limbal lymphatic vessels and corneal lymphangiogenesis. Exp Eye Res. 2008;87:427–432. - PubMed
-
- Van Buskirk EM. The anatomy of the limbus. Eye (Lond) 1989;3(Pt 2):101–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

