Deletion of Osteopontin Enhances β₂-Adrenergic Receptor-Dependent Anti-Fibrotic Signaling in Cardiomyocytes
- PMID: 30897705
- PMCID: PMC6470638
- DOI: 10.3390/ijms20061396
Deletion of Osteopontin Enhances β₂-Adrenergic Receptor-Dependent Anti-Fibrotic Signaling in Cardiomyocytes
Abstract
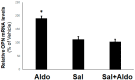
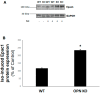
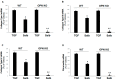
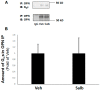
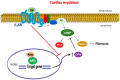
Cardiac β₂-adrenergic receptors (ARs) are known to inhibit collagen production and fibrosis in cardiac fibroblasts and myocytes. The β₂AR is a Gs protein-coupled receptor (GPCR) and, upon its activation, stimulates the generation of cyclic 3',5'-adenosine monophosphate (cAMP). cAMP has two effectors: protein kinase A (PKA) and the exchange protein directly activated by cAMP (Epac). Epac1 has been shown to inhibit cardiac fibroblast activation and fibrosis. Osteopontin (OPN) is a ubiquitous pro-inflammatory cytokine, which also mediates fibrosis in several tissues, including the heart. OPN underlies several cardiovascular pathologies, including atherosclerosis and cardiac adverse remodeling. We found that the cardiotoxic hormone aldosterone transcriptionally upregulates OPN in H9c2 rat cardiac myoblasts-an effect prevented by endogenous β₂AR activation. Additionally, CRISPR-mediated OPN deletion enhanced cAMP generation in response to both β₁AR and β₂AR activation in H9c2 cardiomyocytes, leading to the upregulation of Epac1 protein levels. These effects rendered β₂AR stimulation capable of completely abrogating transforming growth factor (TGF)-β-dependent fibrosis in OPN-lacking H9c2 cardiomyocytes. Finally, OPN interacted constitutively with Gαs subunits in H9c2 cardiac cells. Thus, we uncovered a direct inhibitory role of OPN in cardiac β₂AR anti-fibrotic signaling via cAMP/Epac1. OPN blockade could be of value in the treatment and/or prevention of cardiac fibrosis.
Keywords: CRISPR; Epac1; cAMP; cardiac myocytes; fibrosis; osteopontin; signal transduction; β2-adrenergic receptor.
Conflict of interest statement
The authors declare no conflict of interest related to this publication.
Figures

References
-
- Desimine V.L., McCrink K.A., Parker B.M., Wertz S.L., Maning J., Lymperopoulos A. Biased Agonism/Antagonism of Cardiovascular GPCRs for Heart Failure Therapy. Int. Rev. Cell. Mol. Biol. 2018;339:41–61. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

