Antigen Targets for the Development of Immunotherapies in Leukemia
- PMID: 30897713
- PMCID: PMC6471800
- DOI: 10.3390/ijms20061397
Antigen Targets for the Development of Immunotherapies in Leukemia
Abstract
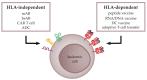
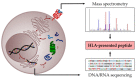
Immunotherapeutic approaches, including allogeneic stem cell transplantation and donor lymphocyte infusion, have significantly improved the prognosis of leukemia patients. Further efforts are now focusing on the development of immunotherapies that are able to target leukemic cells more specifically, comprising monoclonal antibodies, chimeric antigen receptor (CAR) T cells, and dendritic cell- or peptide-based vaccination strategies. One main prerequisite for such antigen-specific approaches is the selection of suitable target structures on leukemic cells. In general, the targets for anti-cancer immunotherapies can be divided into two groups: (1) T-cell epitopes relying on the presentation of peptides via human leukocyte antigen (HLA) molecules and (2) surface structures, which are HLA-independently expressed on cancer cells. This review discusses the most promising tumor antigens as well as the underlying discovery and selection strategies for the development of anti-leukemia immunotherapies.
Keywords: HLA; T cell; antigen; epitope; immunotherapy; leukemia; peptide; target; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Nowell P.C., Hungerford D.A. Chromosome studies on normal and leukemic human leukocytes. J. Natl. Cancer Inst. 1960;25:85–109. - PubMed
-
- O’Brien S.G., Guilhot F., Larson R.A., Gathmann I., Baccarani M., Cervantes F., Cornelissen J.J., Fischer T., Hochhaus A., Hughes T., et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. - DOI - PubMed
-
- Cortes J.E., Kim D.-W., Kantarjian H.M., Brümmendorf T.H., Dyagil I., Griskevicius L., Malhotra H., Powell C., Gogat K., Countouriotis A.M., et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: Results from the BELA trial. J. Clin. Oncol. 2012;30:3486–3492. doi: 10.1200/JCO.2011.38.7522. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

