Time-Resolved Macromolecular Crystallography at Pulsed X-ray Sources
- PMID: 30897736
- PMCID: PMC6470897
- DOI: 10.3390/ijms20061401
Time-Resolved Macromolecular Crystallography at Pulsed X-ray Sources
Abstract
The focus of structural biology is shifting from the determination of static structures to the investigation of dynamical aspects of macromolecular function. With time-resolved macromolecular crystallography (TRX), intermediates that form and decay during the macromolecular reaction can be investigated, as well as their reaction dynamics. Time-resolved crystallographic methods were initially developed at synchrotrons. However, about a decade ago, extremely brilliant, femtosecond-pulsed X-ray sources, the free electron lasers for hard X-rays, became available to a wider community. TRX is now possible with femtosecond temporal resolution. This review provides an overview of methodological aspects of TRX, and at the same time, aims to outline the frontiers of this method at modern pulsed X-ray sources.
Keywords: Monte Carlo integration; bacterial phytochromes; beta-lactamase; serial femtosecond crystallography; time-resolved crystallography.
Conflict of interest statement
The author declares no conflict of interest.
Figures
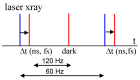


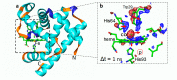


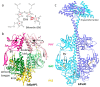
References
-
- Schlichting I., Almo S.C., Rapp G., Wilson K., Petratos K., Lentfer A., Wittinghofer A., Kabsch W., Pai E.F., Petsko G.A., et al. Time-Resolved X-ray Crystallographic Study of the Conformational Change in Ha-Ras P21 Protein on Gtp Hydrolysis. Nature. 1990;345:309–315. doi: 10.1038/345309a0. - DOI - PubMed
-
- Tenboer J., Basu S., Zatsepin N., Pande K., Milathianaki D., Frank M., Hunter M., Boutet S., Williams G.J., Koglin J.E., et al. Time-resolved serial crystallography captures high-resolution intermediates of photoactive yellow protein. Science. 2014;346:1242–1246. doi: 10.1126/science.1259357. - DOI - PMC - PubMed
-
- Pande K., Hutchison C.D.M., Groenhof G., Aquila A., Robinson J.S., Tenboer J., Basu S., Boutet S., Deponte D., Liang M., et al. Femtosecond Structural Dynamics Drives the Trans/Cis Isomerization in Photoactive Yellow Protein. Science. 2016;352:725–729. doi: 10.1126/science.aad5081. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

