Identification of Bis-Cyclic Guanidines as Antiplasmodial Compounds from Positional Scanning Mixture-Based Libraries
- PMID: 30897744
- PMCID: PMC6471430
- DOI: 10.3390/molecules24061100
Identification of Bis-Cyclic Guanidines as Antiplasmodial Compounds from Positional Scanning Mixture-Based Libraries
Abstract
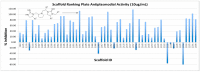
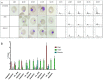
The screening of more than 30 million compounds derived from 81 small molecule libraries built on 81 distinct scaffolds identified pyrrolidine bis-cyclic guanidine library (TPI-1955) to be one of the most active and selective antiplasmodial libraries. The screening of the positional scanning library TPI-1955 arranged on four sets of sublibraries (26 + 26 + 26 + 40), totaling 120 samples for testing provided information about the most important groups of each variable position in the TPI-1955 library containing 738,192 unique compounds. The parallel synthesis of the individual compounds derived from the deconvolution of the positional scanning library led to the identification of active selective antiplasmodial pyrrolidine bis-cyclic guanidines.
Keywords: antiplasmodial; combinatorial chemistry; guanidines; heterocyclic peptidomimetics; malaria; parallel synthesis; solid-phase synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Direct Phenotypic Screening in Mice: Identification of Individual, Novel Antinociceptive Compounds from a Library of 734,821 Pyrrolidine Bis-piperazines.ACS Comb Sci. 2016 Jan 11;18(1):51-64. doi: 10.1021/acscombsci.5b00126. Epub 2016 Jan 5. ACS Comb Sci. 2016. PMID: 26651386 Free PMC article.
-
Pyrrolidine bis-cyclic guanidines with antimicrobial activity against drug-resistant Gram-positive pathogens identified from a mixture-based combinatorial library.Bioorg Med Chem Lett. 2006 Oct 1;16(19):5073-9. doi: 10.1016/j.bmcl.2006.07.037. Epub 2006 Aug 4. Bioorg Med Chem Lett. 2006. PMID: 16890437
-
Identification of two novel, potent, low-liability antinociceptive compounds from the direct in vivo screening of a large mixture-based combinatorial library.AAPS J. 2010 Sep;12(3):318-29. doi: 10.1208/s12248-010-9191-3. Epub 2010 Apr 27. AAPS J. 2010. PMID: 20422341 Free PMC article.
-
Solid phase synthesis of mixture-based acyclic and heterocyclic small molecule combinatorial libraries from resin-bound polyamides.Biopolymers. 2001;60(3):212-9. doi: 10.1002/1097-0282(2001)60:3<212::AID-BIP10033>3.0.CO;2-D. Biopolymers. 2001. PMID: 11774227 Review.
-
Bis(2-aminoimidazolines) and Bisguanidines: Synthetic Approaches, Antiparasitic Activity and DNA Binding Properties.Curr Med Chem. 2017;24(33):3606-3632. doi: 10.2174/0929867324666170623091522. Curr Med Chem. 2017. PMID: 28641558 Review.
Cited by
-
"Cut and Paste" Processes in the Search of Bioactive Products: One-Pot, Metal-free O-Radical Scission-Oxidation-Addition of C, N or P-Nucleophiles.Front Chem. 2022 May 18;10:884124. doi: 10.3389/fchem.2022.884124. eCollection 2022. Front Chem. 2022. PMID: 35665068 Free PMC article. Review.
-
Parallel Synthesis of Piperazine Tethered Thiazole Compounds with Antiplasmodial Activity.Int J Mol Sci. 2023 Dec 12;24(24):17414. doi: 10.3390/ijms242417414. Int J Mol Sci. 2023. PMID: 38139243 Free PMC article.
-
1,5-Disubstituted Acylated 2-Amino-4,5-dihydroimidazoles as a New Class of Retinoic Acid Receptor-Related Orphan Receptor (ROR) Inhibitors.Int J Mol Sci. 2022 Apr 17;23(8):4433. doi: 10.3390/ijms23084433. Int J Mol Sci. 2022. PMID: 35457251 Free PMC article.
-
Polyheterocyclic peptidomimetics: Parallel solid phase synthesis of oligo cyclic guanidines and their inhibition activity against Mycobacterium tuberculosis DNA gyrase.Bioorg Med Chem Lett. 2023 Sep 1;93:129439. doi: 10.1016/j.bmcl.2023.129439. Epub 2023 Aug 8. Bioorg Med Chem Lett. 2023. PMID: 37557925 Free PMC article.
-
Synthesis of Diazacyclic and Triazacyclic Small-Molecule Libraries Using Vicinal Chiral Diamines Generated from Modified Short Peptides and Their Application for Drug Discovery.Pharmaceuticals (Basel). 2024 Nov 22;17(12):1566. doi: 10.3390/ph17121566. Pharmaceuticals (Basel). 2024. PMID: 39770408 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

