LC478, a Novel Di-Substituted Adamantyl Derivative, Enhances the Oral Bioavailability of Docetaxel in Rats
- PMID: 30897775
- PMCID: PMC6471177
- DOI: 10.3390/pharmaceutics11030135
LC478, a Novel Di-Substituted Adamantyl Derivative, Enhances the Oral Bioavailability of Docetaxel in Rats
Abstract
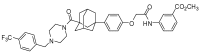
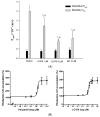
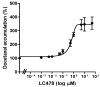
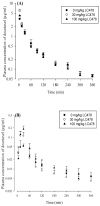
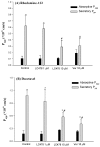
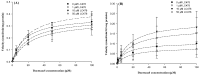
P-glycoprotein (P-gp)-mediated efflux of docetaxel in the gastrointestinal tract mainly impedes its oral chemotherapy. Recently, LC478, a novel di-substituted adamantyl derivative, was identified as a non-cytotoxic P-gp inhibitor in vitro. Here, we assessed whether LC478 enhances the oral bioavailability of docetaxel in vitro and in vivo. LC478 inhibited P-gp mediated efflux of docetaxel in Caco-2 cells. In addition, 100 mg/kg of LC478 increased intestinal absorption of docetaxel, which led to an increase in area under plasma concentration-time curve (AUC) and absolute bioavailability of docetaxel in rats. According to U.S. FDA criteria (I, an inhibitor concentration in vivo tissue)/(IC50, inhibitory constant in vitro) >10 determines P-gp inhibition between in vitro and in vivo. The values 15.6⁻20.5, from (LC478 concentration in intestine, 9.37⁻12.3 μM)/(IC50 of LC478 on P-gp inhibition in Caco-2 cell, 0.601 μM) suggested that 100 mg/kg of LC478 sufficiently inhibited P-gp to enhance oral absorption of docetaxel. Moreover, LC478 inhibited P-gp mediated efflux of docetaxel in the ussing chamber studies using rat small intestines. Our study demonstrated that the feasibility of LC478 as an ideal enhancer of docetaxel bioavailability by P-gp inhibition in dose (concentration)-dependent manners.
Keywords: LC478; P-glycoprotein; absorption; bioavailability; docetaxel; inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- van Waterschoot R.A., Lagas J.S., Wagenaar E., van der Kruijssen C.M., van Herwaarden A.E., Song J.Y., Rooswinkel R.W., van Tellingen O., Rosing H., et al. Absence of both cytochrome P450 3A and P-glycoprotein dramatically increases docetaxel oral bioavailability and risk of intestinal toxicity. Cancer Res. 2009;23:8996–9002. doi: 10.1158/0008-5472.CAN-09-2915. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

